As discussed here extensively, nothing in “evolution” makes any sense: “natural selection, fitness, speciation, human evolution, gradualism, divergence of character, UCD, TOL, etc. etc.” Not one makes sense. Meanwhile, the “evolution” argument is just one big “affirms the consequent” logical fallacy, while Paley’s excellent argument has never been overturned, and an intuitive intelligent design detector can be used to easily disprove “evolution”. Is there a need for any more proofs? Not really. Are there any other proofs? You bet. Take entropy for instance…
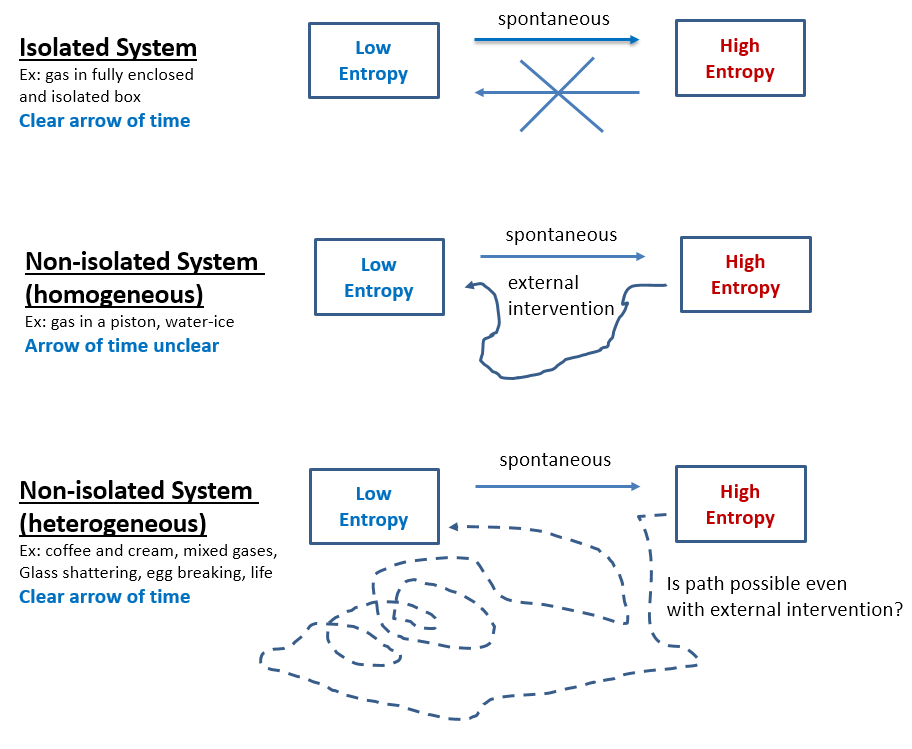
Figure 1
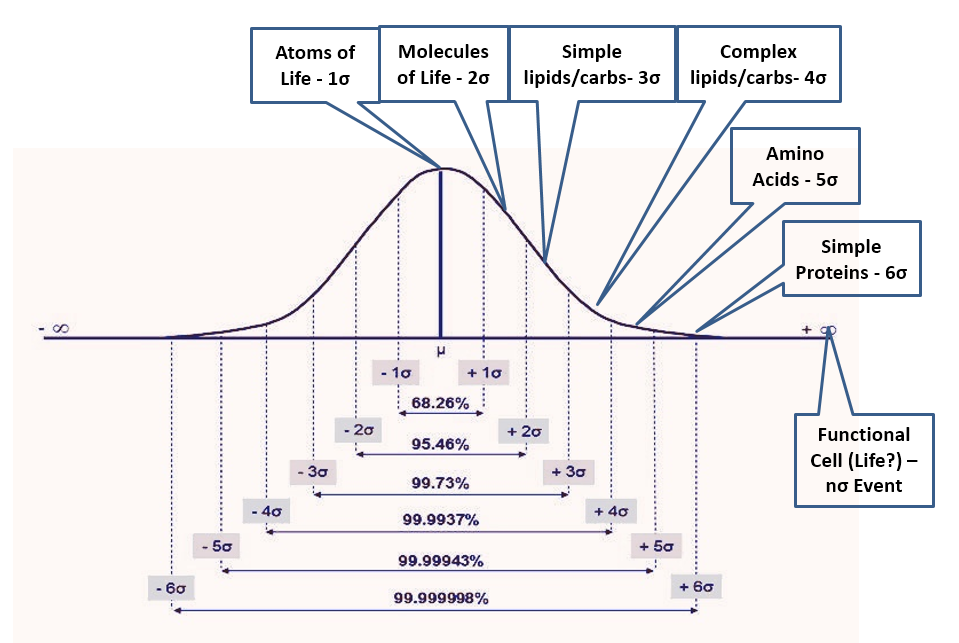
Figure 2
- Second Law of Thermodynamics shows that a spontaneous process cannot also revert spontaneously. This is because spontaneous processes always increase the system’s entropy. A uniform gas in a chamber will accumulate in a corner only with external intervention and spontaneous chemical reactions can only revert if external work energy is applied. Current models of entropy assume the gas particles in a chamber to be independent (sometimes represented as pebbles on a Go board) and explains their never observed convergence on one side of the chamber as only due to that particular microstate having a very low probability(*). However, gas particles always interact with each other (Brownian motion) while pebbles do not. Thus, a reliable way to know that entropy of a system increases is if work energy could be obtained when transitioning from the low to the high entropy state while energy is always required for the reverse process.
- Total entropy of an isolated system can never decrease. Entropy is currently assumed just a statistical law. Thus, if N molecules are in an isolated system (box), the number of microstates associated with j of them being in one half while N-j being in the other half is Ω = N! / (j!*(N-j)!). If N is small, fluctuations seem possible, but before N increases to anything measurable, the probability of fluctuations rapidly decreases to nil. Furthermore, even these theoretical fluctuations, as improbable as they are, might be impossible since the statistical view does not account for molecular interaction observed as Brownian motion and as gas resistance to compression and expansion. Better fundamentals or statistics, either way entropy will never decrease spontaneously in an observable system (Fig 1.a).
- Decreasing entropy is not the reverse process of entropy increasing. That is why a broken egg coming together is easily identified as unreal and a reversed movie of its real shattering. The known laws of physics are the same forward and backward (time-reversal invariance), therefore the reverse shattering process of an egg would not violate any law, but only because these laws are always idealized. Supposedly, if just the right forces are applied to the broken pieces, the egg will come together. In reality this is impossible, and not because the unbroken egg is a highly unlikely microstate, but because entropy increase is not directly reversible even in non-isolated systems. This irreversibility holds for all heterogeneous systems, including life which is perhaps the most heterogeneous system of all. Entropy increase is directly reversible only for homogeneous systems and only if in a defined space. For instance, an expanding gas in an ideal piston creates a force that, when reversed, compresses the gas back into its original state. However, a solid cube of ice can be easily melted by increasing the temperature, but the original ice cube will not reconstitute by lowering the temperature, hence this process too is irreversible despite the cube of ice being homogeneous (Fig 1.b). As far as heterogeneous systems, even separating two mixed gases is way different than the original mixing process, hence mixing is irreversible (Fig 1.c). Entropy decrease is not only different, but also much more complex than entropy increase which is usually spontaneous. Abiogenesis is the entropy-lowering reverse of the biologic decay process, and therefore – if at all feasible – much more complex than adding chemicals and energies.
- Once in equilibrium, a “primordial soup” does not change spontaneously. Life is metastable – it requires certain forms of energy to sustain and spontaneously decays when it no longer receives that energy as well as after the end of the normal lifespan of the organism. It was hypothesized that random fluctuations can spontaneously create compounds and structures given enough time. Abiogenesis, as a reverse-decay process, cannot simply be an outcome of Brownian motion of the chemicals mix because a perpetual motion machine powered by decay and abiogenesis cycles would violate the ‘conservation of energy’ principle. Experimentally, one can confirm that chemical blends in static equilibrium never transition spontaneously into a different equilibrium state (this includes oscillating reactions after the settlement period).
- A “primordial soup” cannot generate life even if energy is applied. It was hypothesized that abiogenesis can be a product of tidal pools, deep sea hydrothermal vents, and the undersurface of ice caps where persistent and abundant energy is available in the form of thermal and electrochemical gradients. Indeed, energy can throw systems off balance and create all kind of chemical compounds and physical structures. However, as the energy applied increases, a complexity limit and hence a dynamic equilibrium is reached where molecule destruction offsets their creation and, if even more energy is applied, molecule destruction dominates, eventually leaving the experimenter with gunk and none of the desired molecules. Miller–Urey and subsequent experiments were not ended because they reached their goal – life – nor because they ran out of energy and materials, but because they reached this dynamic equilibrium, and by adding more of anything would have left them with fewer of the targeted compounds. The amino acids obtained were not the end product but the intermediate between the original molecules and the useless gunk that was the product of the Maillard reaction caused by the energy applied to the system. More complex molecules (and maybe life itself one day) can be created by intelligent designers adding targeted compounds and energies. Then “why can’t natural processes somewhere somehow just mimic the intelligent designer in this vast and almost timeless universe?” The better question is: “why insist on natural processes when the model to be mimicked is that of the intelligent designer?”
- If natural processes were capable of generating life, the environment would be full of intermediate bio-compounds. Life is so complex that laboratories have no hope of replicating it in the foreseeable future. However, if abiogenesis were an outcome of natural processes, the cell structure would be produced only from subsystems and complex biomolecules that in turn would depend on simpler molecules down to H-C-O-N, the atoms of life. A “primordial soup” capable of generating life, thus must contain all intermediate compounds from the atoms of life to the most complex biomolecules and subsystems in an ever-decreasing ratio as complexity increases. Not knowing anything about how this process would work (or even if possible), the most reasonable assumption is a normal distribution of outcomes with life being an n-sigma event (with n unknown) while the availability of the atoms of life being a 1-sigma event and anything else falling in between (Fig 2). Many x-sigma events would be required for each (x+1)-sigma event, with a good first approximation given by the normal density function. Thus, the 2-sigma event could be the basic molecules of life (water, methane, etc.), and we would expect only one of these events for every seven of the 1-sigma events. This approximation would further yield (in one scenario) 1/7 fewer molecules of life than atoms of life, 1/17 fewer simple lipids and carbohydrates molecules (3-sigma) than of molecules of life, 1/43 fewer complex lipids and carbohydrates (4-sigma) than 3-sigma events, 1/110 fewer amino acids (5-sigma) than 4-sigma, 1/291 fewer simple proteins (6-sigma) than 5-sigma, 1/771 fewer complex proteins (7-sigma) than 6-sigma and then – rule of thumb – 1/1600 (8-sigma), 1/3800 (9), 1/9100 (10), 1/22k (11), 1/52k (12), 1/126k (13), etc. fewer of each additional sigma event than previous event where 8+sigma being (this scenario) nucleic acids, short chains, long chains, organelle subsystems, organelles, other critical cell components and finally the fully functional biologic cell – the n-sigma event which is not quite life but good enough for this analysis. Then how can we test this?
- Apart from life itself, the complex molecules of life are nowhere to be found in the universe. To test the ‘natural processes’ hypothesis of abiogenesis, one must observe the intermediate components of life in nature and in the ratios estimated above (or from another reasonable estimate). In addition, one must observe the spontaneous transitions (aided by energy) from simple to complex even if not all transitions are observed at once. Earth is “polluted” with life down to the deepest ocean trenches, therefore the first focus is the extraterrestrial space where, too bad, the largest confirmed interstellar molecules have a maximum of 13 atoms (apart from C60/C70 fullerene). Back on earth we see all intermediate components, but only within life itself. Outside of the cells, aside from the simplest biomolecules, we only see products of decomposition that are never in the ratios associated with abiogenesis, meaning we never see increasing molecule complexity in decreasing ratios resembling anything reasonably expected. Abiogenesis is not happening due to the irreversibility of the entropy increase and for the same reason egg breaking, butter melting, gas mixing, etc. are not reversible processes. Humans can only create a few of the complex molecules, although most always aided by life itself, and even then the power of synthetic biology is severely restricted. The more complex, the harder these molecules are to obtain and the faster they decay instead of spontaneously combining with one another to form even more complex compounds and ultimately life.
- Miller–Urey style abiogenesis experiments are ill conceived, hence doomed from the beginning. To be more specific, they are only good for PR (public relations) given the irrelevant “organic compounds” created that raise the hopes of the believers. Trying to obtain an automobile from scratch by mixing chemicals and energy, qualifies the person attempting as delusional and the one selling such vision as charlatan. So why would those attempting the same with life – which is infinitely more complex than an automobile – not also be labeled charlatans and delusional? Abiogenesis experiments belong to the Reverse Engineering category of processes and, when done right, they are very different than Miller–Urey. Their starting point is never some “primordial soup”, but the most advanced compounds available, preferably already organized in working subsystems. Swapping organelles or parts within organelles, exposing organisms to various environments, attempting to revive dead organisms, substituting engineered subsystems and so on are part of the hard work with long tradition and already being done in medicine and many industries for other purposes than to prove abiogenesis. If and when someone will be able to reverse the decaying and dying processes, we will know that abiogenesis is possible as an act of Intelligent Design creation. To confirm abiogenesis as an “unguided process” we would have to observe reverse-decay and reverse-dying processes happening in nature, not in a lab. Yet 2nd law proves this impossible.
- Is abiogenesis not feasible because it was a unique event? If true, abiogenesis would be a “materialistic miracle” and furthermore not just one, but a long series of “materialistic miracles” since a long series of – so far unknown – events are needed to get from atoms to the simplest organism. Yet one of the tenets of materialism is “no miracles” showing the inconsistency of the materialistic “unique event” assertion. And of course, physics and chemistry transformations are never unique. And even if entropy allowed for abiogenesis, the laws of life do not follow from any priors (physics, chemistry, mathematics). Life has a drive to survive and leave off-springs which entails harm avoidance, immune system, metabolism, food seeking, homeostasis, growth, reproduction, and body structure. Without these, any cell would start decaying the instant it was formed as in fact it does as soon as it no longer is alive. Despite having lasted almost since the formation of The Earth, life is metastable – one knock and it dies and then decays. This is unlike other negative entropy machines that can be restored (rebuilding proportional with the damage).
- Other considerations.
- “Dissipation-driven adaptation of matter” (J. England, MIT) claims that life is inevitable because life “absorbs and dissipates more energy from external sources” leading to faster entropy increase. However, there is no law that entropy has to increase faster. In addition, most of the entropy in the universe is captured by black holes with life having a nil contribution to that entropy.
- Some claim they have obtained “protocells” that seem to mimic real cells at least in part. However, “protocells” are to biological cells as fool’s gold is to real gold.
- “Kolmogorov complexity is lowest at low and high entropy and high in the middle hence life is supposedly inevitable (S. Carroll)”. However, life is not complexity. Life is much more than snowflakes, vortices and chemical reactions (candle burning). And most certainly, life is not the complex swirls of cream mixing into coffee on a journey from low entropy to high entropy (both having low complexity). In addition, unless very specific external action continues to be applied to maintain those patterns, they soon disappear like in sand dunes exposed to shifting winds. The patterns therefore do no “arise”, but are created by an external force.
- “Gradients of energy in deep vents are responsible for abiogenesis”. But all organisms from these exotic places are very similar to any other ones found elsewhere, hence all likely have the same origin. In addition, no free floating organic compounds (aside from decay byproducts) have been found there to suggest ongoing abiogenesis. And, aside from the simplest molecules, no spontaneous transitions from x-sigma to (x+1)-sigma bio complexity has ever been observed around these deep vents either.
- Of course life does not violate 2nd Organisms do conform to 2nd law when they decay as soon as they die. In addition, as observed by Erwin Schrödinger, “the increase in entropy from turning our low-entropy food into our high-entropy waste is greater than the local decrease in entropy from making the well-ordered structures within our bodies”. Nothing special so far – a refrigerator does the same: creates a zone of low-entropy while the entropy of the whole system increases and for as long as it’s fed energy.
- Randomness can theoretically account for any bizarre occurrences including Paley’s watch and F. Hoyle’s 747 in baby steps if enough time is given. But no such event was ever observed. In addition, breaking down the unattainable complex system into a combination of simpler components, each with higher probability of occurrence makes it no easier as the probabilities of all subsystem have to be multiplied to get back to the complex final assembly.
- Some claim that life itself prevents abiogenesis by ingesting all intermediate molecules spontaneously formed, but this can be easily prevented in sterile labs. In addition, all complex intermediate molecules observed outside of cells are due to decomposition, not abiogenesis.
- “Evolution” corollary number 1. If abiogenesis is impossible as an undirected, natural process, then whoever is responsible for abiogenesis is also responsible for the biologic landscape past and present, therefore “evolution” is also impossible as an undirected, natural process.
- “Evolution” corollary number 2. It is easy to verify that nothing ever “evolves” in the nonliving nature. Life is said to be “just chemistry”. These two combine to: nothing “evolves” in the living either. Solar systems, geographical features, fluid eddies, chemistry, snow flakes, etc. all go through their life cycles, and all are different from each other, but the life cycles of the newer entities are no more “evolved” than the life cycles of the ancient ones.
- “Evolution” corollary number 3. Presumably, “evolution” has not ended. And if ongoing, then one must see the normal distribution of the different transitioning organisms (the intermediary), just as we would see if abiogenesis were true. If humans evolved from monkeys and “evolution” is ongoing, then humans must still be in transition especially since the human population is one of the largest of all mammals and, the more individuals, the more “evolving” opportunities. The older Darwinists replied with a hierarchy of races. But that reply is not only fashionably repugnant, but also false and, amazingly, contrary to [at least] the Abrahamic religions that have always known better.
- In conclusion, abiogenesis is nothing more than the decay process running backwards, therefore easily visualized, yet impossible according to the second law of thermodynamics. In other words, “evolution” is nothing more than imagination run wild. Expecting abiogenesis to be within reach if only the proper forces and chemical compounds were added is as wrong as expecting the broken egg to come back together if only the proper sequence of forces were applied to the broken pieces.
Summary:
- A spontaneous process cannot revert spontaneously.
- Mixtures will never ever spontaneously separate per second law.
- Decreasing entropy is not the reverse process of entropy increasing and also much more complex.
- Once in equilibrium, a “primordial soup” does not change spontaneously.
- A “primordial soup” cannot generate life even if energy is applied due to dynamic equilibrium.
- If natural processes were capable of generating life, the environment would be full of intermediate bio-compounds.
- Apart from life itself, the complex molecules of life are nowhere to be found in the universe.
- Abiogenesis experiments belong to the Reverse Engineering category of processes.
- Miller–Urey style abiogenesis experiments are ill conceived, hence doomed from the beginning.
- Abiogenesis unique event conflicts with the “no miracles” clause of materialism.
- Even if entropy allowed abiogenesis, the laws of life do not follow from any priors (physics, chemistry, mathematics).
- “Evolution” corollary number 1 – no abiogenesis, no “evolution”.
- “Evolution” corollary number 2 – no “evolution” in the inert and “life just chemistry”, then no “evolution” in the living.
- “Evolution” corollary number 3 – no intermediate “evolving” entities, no “evolution”.
- Being a decay process running backwards, abiogenesis is as impossible as a broken egg being reconstituted by the “proper sequence of forces”. “Evolution” is also nothing more than imagination run wild.
(*)R. Penrose “The Emperor’s new mind”; PBS SpaceTime “The Misunderstood Nature of Entropy”; Sean Carroll “From Eternity to Here”, etc.
Links:
Abiogenesis: The Faith and the Facts
James Tour: The Mystery of the Origin of Life
Chirality, Maillard – caramelization, characterize the structure at every step:
https://creation.com/why-the-miller-urey-research-argues-against-abiogenesis
https://evolutionnews.org/2014/06/squeezing_the_l/
https://www.ncbi.nlm.nih.gov/pubmed/21422282
Entropy of a box of molecules
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2253472/ https://en.wikipedia.org/wiki/Normal_distribution#Cumulative_distribution_function
http://physics.bu.edu/~redner/211-sp06/class-engines/class25_secondlaw.html
https://www.quora.com/How-quickly-is-the-entropy-of-the-sun-changing
https://www.thoughtco.com/how-many-atoms-in-human-cell-603882
https://www.amazon.com/Mysteries-Modern-Physics-Sean-Carroll/dp/1598038699
https://en.wikipedia.org/wiki/File:Elements_abundance-bars.svg – abundance in the solar system
https://en.wikipedia.org/wiki/Miller%E2%80%93Urey_experiment
https://en.wikipedia.org/wiki/Proteinogenic_amino_acid
https://en.wikipedia.org/wiki/Artificial_gene_synthesis
https://www.scientificamerican.com/article/a-new-physics-theory-of-life/
https://en.wikipedia.org/wiki/Dissipative_system
https://pubs.usgs.gov/gip/dynamic/exploring.html

Such nonsense, Alan.
Why does COVID kill? Science can’t say. Why is it better to wear masks? Science can’t say. Why is social distancing important? Science can’t say.
Why can anyone post nonsence on TSZ? Science can’t say!
So much for Scientism, which dominates this site.
Science cannot explain everything because Science is largely ignorant. Science can only explain what Science can explain. Science is incapable of telling us what Science can and cannot explain. Have faith.
Why should phoodoo have to volunteer?
You see phoodoo, DNA_Jock and Alan collaborated to freeze a live human being, attempted to freeze-thaw that same human being, and failed. They then determined that “ice-crystals” could be blamed for their incompetance.
Now they want you to agree to submit to whatever procedure they previously employed, which they readily admit was a failure. All in the name of Science!
I mention how I found that and Nonlin could not check it herself. To be expected though, given Nonlin’s astounding illiteracy.
100% of what? Of whatever those geneticists were working on, obviously. It’s not difficult Nonlin, you could do it yo … ooooo … no, you couldn’t. Sorry, I keep forgetting that your illiteracy is severe. Simple arithmetic might be way too much for you.
Nature is always looking for the things that might change everything. Whatever might shape science in the years to come. “Darwinism” would be a tad outdated for them. I insist, though, that if you’re from the US you check Science Magazine instead. They’ll be glad to beat Nature in finding Nonlin the prodigy. The ten year old who changed everything we know about both thermodynamics and the origin of life.
🤣
Sure, we do. So, what’s the difference in entropy between an entire and a broken egg?
Indeed – people who understand the subject matter. You need to concentrate your fire on people who don’t have a clue, perhaps. It’s a Conspiracy of The Knowledgeable. Because anyone who understands entropy would laugh your bullshit out of court. So you are reduced to trolling backwaters, a prophet without honour. Sucks to be you.
What about it?
Even if I was the only guy, the statement would still be true. The frequencies of the instrument categories in an orchestra also add up to 100%. Which orchestra? Doesn’t matter. Have I checked? Don’t need to.
Neither are you. Ya boo sucks, I know you are but what am I, etc. Stunning rebuttals all.
So the fridge works harder when the door is closed than open? Hahahaaaa! I’ll save ££££’s keeping it open, then. Top tip.
Indeed, thanks for the lesson; I had no idea solutions were different from pure molten substance. That’ll teach me to listen in class.
Ask your repair guy why crystals form, in both solidification and precipitation scenarios, without the need to ‘apply energy’. (Joe G’s a fridge repair guy, I believe …)
Also very dumb. You can hit saturation by adding solute, as well as removing solvent.
Wrong. Crystals precipitating out do not have ‘lower local entropy’. The energy of the uncrystallised substance has departed. It has shuffled off this mortal coil. It is ex-energy (OK, not really, 1st Law and all that, but poetic license in service of the joke). Entropy is a measure of the capacity to do work. That involves energy. The energy departs (as heat), and the result has less capacity to do work. ie, greater entropy.
Of course another change is dissolving those very same crystals. If you increase the concentration of solvent, this will also happen spontaneously. Now, it is the ‘dissolved’ state that has the higher entropy. The exact same crystal has higher entropy than the saturated solution – it won’t dissolve spontaneously in it – but lower entropy than the unsaturated solution. Fiddle with temperature, you change that relationship again.
Not just a mega-problem for abiogenesis; a mega-problem for your continued existence, if your analysis was accurate. You should spend less time talking to fridge repair men and more time talking to biochemists. I’m a biochemist. AMA.
Your examples – eggs breaking, etc – are entirely drawn from the viewpoint of ‘entropy as order’. As is your error in thinking crystals intrinsically have lower entropy than their dissolved form. You don’t have to say ‘order’ for it to be obvious you are mistaking the illustrations for the underlying concept. Map-territory confusion, as Jock put it.
Fructose. Check out a jar of clear honey after a few months. You can reverse the process by heating (increase entropy). Returning to room temperature, it recrystallises spontaneously (which does not mean instantly).
So you don’t know if they were expected to directly generate life or not? And yet you are sure that enough has been done to eliminate the possibility. 🤔
The documentation of failed research? I call bullshit; an unsupported claim.
I missed that. And I don’t believe you. You are now setting yourself at odds with statistical mechanics, claiming ‘evidence’ that statistical reversals are more likely in non-equilibrium situations than at equilibrium.
Why thank you. I do my best.
Science (using as synecdoche) can’t explain why Covid19 kills, It is very effective at enabling us to understand how it functions and how we can combat the spread of infection.
Social distancing works by limiting the spread of the virus (and could have a huge impact on the prevalence of other respiratory viruses such as influenza) but it also is having a negative social impact on people’s lives such as mental health, education, economic stability.
Imagine a world-wide effective, comprehensive lockdown and international travel ban held in force for 30 days at the beginning of this year. Why didn’t Trump encourage that? Science can’t answer.
Indeed, science plays no rôle in the moderation policy here. Overwhelmingly, what turns up gets published.
Weeeeelll … matter is principally of thermodynamic interest as a carrier of energy. When people in top hats were fretting about the loss of efficiency in steam engines, they weren’t so much wondering where all the matter went…
It isn’t. It’s a measure of the capacity to disperse energy, or, the same thing, to do work.
I’ll give you one guess…
That is much to vague to have any useful meaning. Each plant will have its own pulsating rhythm where the life force ebbs and flows. The life force in plants such as brambles and stinging nettles retreats into the roots as winter approaches and this is easily seen as they die back towards the ground as winter comes along. With annual plants the strong life forces of spring are given up and they become concentrated in the seeds through which the life of the plants continues further.
Materialism is a necessary stage of human culture. The feeling that we are insignificant creatures on a lonely little planet which is nowhere in particular ensures that we stand on our own two feet and learn to fend for ourselves. It’s like when a child learns to ride a bike with a parent beside them holding onto them. When the parent lets go the child might feel as though s/he’s been abandoned, this is how they learn.
We must find our way back to our roots by our own effort. The roots of a plant are attracted to the centre of the earth. Our roots lie in the opposite direction.
CharlieM,
It was an ironic reflection of your own stance, so any such issues relate back to the source material.
Scorpions have more life-force than wood frogs, in the desert. In Alaskan winters, the position appears reversed. Curious, huh? Perhaps it’s not an intrinsic property?
Thank goodness you avoided anything vague! 😉
You haven’t given any alternative explanations. You have pointed out specific, recognised differences between us and amphibians and expect me to believe that they are causes in themselves.
CharlieM,
I gave three mechanistic explanations, only tangentially related to “the differences between amphibians and us”. You don’t like them, I have others, as Groucho would say. All of them, you boil down, without evidence, to the expression of Life Force. It’s a quality that explains everything, therefore nothing. The proof of its existence is existence.
Is something alive, despite environmental challenge? Proves it has Life Force. That is the depth of your argument here.
Freeze a newt, see what happens. Try an Amazonian frog. Are you sure the resistance of wood frogs to freezing is all down to their being amphibians; those ‘regenerative powers’?
Meantime, who’d have thought an organism out of Africa would fail to come out of freezing unscathed? It’s a puzzle an’ no mistake.
From here
It might be better, in order to reduce disorderly confusion, if those discussing entropy qualified its use by stating what specific entropy they were talking about. Not only is it a physical condition it seems that it is also a person 🙂
I gave up smoking over three decades ago. And even then I stuck to good old standard tobacco. The stomach can deal with solids, the lungs can’t. Hopefully my lungs have regenerated enough not to have been notably damaged in a lasting way.
Paradoxically ‘Entropy’ is trying to order my life. 🙂
Hey Nonlin, do you believe that homeostasis to some degree applies to all levels from cell nuclei, to life as a whole? And there are degrees of homeostasis between extant life forms? Are these patterns that aligns with your thinking?
Allan Miller,
Small correction – the crystals in honey are mainly glucose, despite fructose being the main carbohydrate. Fructose will crystallise; just less readily.
You tell me. Are you going anywhere with this? We know a broken egg has higher entropy.
In your own mind, you’re a champ.
Sure, as long as you don’t want any ice and local entropy decrease in general.
What happened to “spontaneous”?!? You’re losing track of your own nonsense.
This is extremely retard. Is heat the ONLY energy form you know? And anyway (hot or cold), biofuel does a whole lot of work even in your car, so obviously the cell is much lower entropy than the sum of its base molecules.
And the problem with “capacity to do work” is that it’s hard to evaluate and compare. What’s wrong with Boltzmann’s formula?
You’re saying that gram-for-gram ice does not have lower entropy than water? Go find another lost soul that supports that nonsense.
Confused nonsense. Last I checked, dissolved crystals ALWAYS have higher entropy than their equivalent solid crystals. Period. You have yet to prove otherwise. No ifs or buts.
Prove it!
If you’re a biochemist, then biochemistry is in big trouble. Try learning a skill. Perhaps repair technician. Nah, entropy keeps tripping you.
False. I go by Boltzmann’s interpretation – micro-states compatible with a macro-state. “Order” is not part of that formula.
And anyway, I asked you to comment on the entropy of those states: broken vs intact egg (and the cell in general). Which you can’t or won’t because it’s inconvenient. But obviously the entropy of those states is different from each other and it’s obvious which way the arrow of time goes.
This is FALSE. Honey crystallizes because it is a super saturated solution: https://www.bjcp.org/mead/crystal.pdf
Crystallization is slow, but NOT spontaneous as the water from the solution has been evaporated as a precondition and thus the total entropy of the initial solution has increased already (when including the water vapors). No mystery, and no entropy reversal there. Heat indeed increases the temperature, but bringing back to room temp REDUCES entropy, hence the recrystallization. No magic entropy reversal there. WTF?!?
Meaning? Translate this gibberish.
The rest of your comments are just stale BS.
Einstein thinks matter is energy. Go figure. Of course, no one has any freaking idea what either of those is. But keep yapping about “energy”.
Boltzmann’s interpretation works fine for now. Of course, other than “evolution” being such an obvious and DEMONSTRABLE retard cretinism, no one knows anything about anything else FOR SURE.
Sorry, I don’t do well with poetry. But yes, ABSOLUTELY ALL organisms do homeostasis. Last I checked… Different methods…
Three of the points in your summary from the op are:
I agree. If the physical (material aspect) is not prior then maybe the life principle is prior and all non-living matter is a by product of life. It is possible that material substance which is subject to the pointwise force of gravity has condensed from the peripheral field-like life principle. The polarity of point-wise and peripheral planar forces giving rise to living matter.
I agree that there has been no spontaneous production of life from non-life. I class that as a miracle believed by followers of the modern day creation story. Maybe life from non-life is for all intents and purposes impossible alternatively, maybe non-life from life is entirely feasible..
The life principle is conceived as being totally organised and thus it is the organising principle. The cycle of growth and decay is observed as the rhythm of the dynamic processes resulting from the interaction of the opposite polar forces, the material aspect and a life principle. Anabolic and catabolic processes are features throughout the cosmos at all levels.
Well the life principle is not an amorphous property, that is certain. The physical attributes of each are an expression of the life principle integral to each. The life principle is just as individual and even more complex than the physical substances that make up each organism. You are in error if you think of it as being similar to the relatively simple lines of force pictured in a magnetic field.
Put a wood frog in the desert and it won’t be long before you see its life force irreversibly draining away. Put a scorpion in a freezer and its life force will definitely diminish but it need not be irreversible.
Who do people listen to when they ask about our ultimate origins? Cosmologists, perhaps? People generally believe our roots lie in the wide universe. Humans send out searching probes to explore the cosmic spaces. Plants being tied to the earth send their roots into the earth to sustain their lives.
What is your opinion about humans being upside down plants? Do you agree with Darwin that roots are like the brain of a plant?
Giving mechanistic explanations is one-sided and ignores the polarity inherent in living systems. It is the application of thinking borrowed from Newtonian physics which only deals with on half of reality. It is like restricting geometry to that of Euclid while failing to comprehend that this is just a static instance of a much more inclusive projective geometry.
Darwin focused on the pointwise Newtonian pole in his quest to understand origins. Prior to Darwin, Goethe had recognised the polarity inherent in nature.
Lenoir notes:
Unless you recognise the peripheral pole, which is the complement of the pointwise pole, the life principle will not enter your field of (inner) vision. There will be no forthcoming, “I see what you mean”. It does not suffice to theorise about the life principle, it has to be individually ‘seen’.
This polar aspect does away with the need for abiogenesis. Life does not spontaneously appear from nowhere, it condenses out of the wide spaces just as solar systems condense out of fields of energy.
And the development of each new organism is a microcosm of the same process. Matter is built up from below into bodies which take their form from above. The building up of matter is achieved through genetics.
Even observing a physical plant it can be seen that the roots gather the material from the earth while the sun draws the shoot system towards as it spirals upwards. The earth provides the materials the heavens provide the form.
Nonlin.org,
Yo’ve descended into a spiral of nonsense, so let me give you a summary:
1. Looking around makes it obvious that the second law of thermodynamics doesn’t “forbid” local increases in entropy. More importantly, that it doesn’t “forbid” building a whole organism from molecules to cells to organism, etc. Therefore, if there’s any problem for abiogenesis, it’s not the second law.
2. Your insistence that abiogenesis is “reverse-decay” is nothing but your unfounded and indefensible claim. We don’t need to convince you otherwise, the burden of proof is all yours. While trying to defend it, other than by tantrums (as if you could!), make sure you can also explain why scientists interested in the origin of life are looking for places and circumstances where there’s energy available for work, at least in bursts, rather than just staring waiting for “the movie” to “run in reverse”.
3. Since you stopped answering me after it was too obvious that you were contradicting yourself: I win!!
To say that ‘resistance of wood frogs to freezing is all down to their being amphibians’ is no explanation. We should be asking why creatures such as amphibians in general have greater powers of regeneration than higher vertebrates do. It is for the same reason that my liver has greater powers of regeneration than my brain.
Amphibians are closer to the earthly pole than, say, birds which are more biased towards the heavenly pole. The liver, being below the diaphragm is closer to the earthly pole than the brain. Both amphibians and the liver have retained a greater ability of growth than birds or brains.
But for this to satisfy you as some sort of explanation you would first need to see the polarities in nature as a reality.
With evolution as with entropy everything depends on how these terms are defined. Evolution as change over time is demonstrably true.
I didn’t ask about all organisms, I asked about all levels, both above and below the organism.
I don’t know and am reluctant to speculate. Raising the issue is enough.
Once again, let’s stay closer to the common meaning. Those concepts refer strictly to biology.
I was wondering when Goethe might show up… 🙂
Your theory is as good as undirected abiogenesis. Now all you need is some sort of proof.
You mean “decreases”. No, just abiogenesis as an “undirected process”.
Building by God, true. Maybe even by some future humans. Maybe! As “undirected process”, impossible. Theoretically AND experimentally.
Furthermore, even the smallest entropy decreases can and MUST be traced to a driving factor. When we see water freeze and melt, we trace that specifically to the rotation of the earth and heat from the sun. It’s never “just happens” or “the universe mysteriously does that”. To scientifically inclined curious minds that is.
Abiogenesis is NOT. It is only imagined. That imaginary film looks like “reverse-decay”.
Decay: cells and complex molecules break apart to form simpler molecules. Abiogenesis: simple molecules come together (imaginary) to form complex molecules and cells.
As explained, energy is not the issue. The asymmetry of entropy changes IS.
Those guys are looking under the lamppost. No wonder they find nothing.
Have a cotton candy on me, winner.
For more about entropy, this guy is pretty good: https://www.youtube.com/watch?v=K36Rb50jRCI
https://www.nasa.gov/feature/goddard/2020/pristine-space-rock-offers-nasa-scientists-peek-at-evolution-of-life-s-building-blocks
It seems that complex molecules are literally falling out of the sky. Did your magic man put them there?
Very interesting that you thought that repeating your refuted-ad-nauseam nonsense would result in a different outcome.
So you can read sometimes? Sure, local decreases. Not “forbidden”, therefore entropy is not a problem for abiogenesis. Simple, right? Undirected? You know that lots of natural phenomena have directions, right?
A mere unsupported and self-refuting claim. Sorry, you fall again into your own trap, the one you ignore every time I make it explicit, for obvious reasons:
a. The magical being interferes, without leaving any traces of Her doing so, all the time against the second law, therefore we would see things going against the second law and we’d be unable to even suggest that there’s a second law at all. No second law, nothing there to “forbid” abiogenesis.
b. What textbooks and experiments show is true and molecules-to-cells-to-organisms works without interference by magical beings, and according to the second law. Therefore entropy is not a problem for abiogenesis.
What the hell are you talking about? It happens all the time with no human intervention. Look around you!
Undirected? Again, lots of natural phenomena have directions, take entropy for an example. Impossible? We see it happening all the time. Therefore you’re ill informed about both theory and experiment. Lots of experiments and theory in biochemistry show that all of it works according to the laws of thermodynamics. Sorry, reality trumps your evidence-free assertions.
So you think that abiogenesis is proposed to have happened in a non-rotational, no-heat-from-the-sun, Earth? That’s yet another new level of nonsense for you. A brand new record!!
🤣
Reproduction: simple molecules come together to form complex molecules and cells. You’d be hard pressed to define reproduction as “reverse-decay”, you did that time and again, until you quitted and made your definition abiogenesis-specific for no reason but your own preference. It’s still just an indefensible claim. See? No evidence, nothing but your mere say so.
As explained, energy is the issue. We’re talking about open systems Nonlin, not about the irrationalities of your imagination.
Now, asymmetry? Didn’t you just declare that everything in nature is “undirected”? If so, then there would be no asymmetry, entropy would run in either direction willy-nilly. Either that or you admit that natural phenomena have directions. You lose either way.
Very strange way to admit that scientists are not looking for “reverse-decay.” But I take your admission of defeat. Now that you understand it’s just you, be a good girl and stop your “reverse-decay” nonsense. It makes you look both illiterate and astoundingly stupid.
Given your illiteracy, no wonder that you miss what they find.
I don’t eat cotton candy. Your preferences, like your ignorance, are yours Nonlin. Stop projecting.
See? As expected, nothing but your unsupported and indefensible claims. Good thing you decided to concede that you were wrong about what scientists are looking for. Not before submitting more exemplars of your extraordinary nonsense and breaking your old records.
Thanks again for the entertainment.
I like your philosophy 🙂
Obviously in speaking about the whole cosmos, I’m using them in the more general sense of building up and throwing down.
Gone but not forgotten 🙂
It’s more of an inner observation than a theory and everyone makes their own observations.
CharlieM,
I said nothing of the sort! Newts and Amazonian frogs are also amphibians. You can’t freeze and revive ’em successfully. There is something specific about wood frogs in Arctic regions, and it isn’t ‘they are amphibians’!
I despair.
CharlieM,
Yikes. And this beats having a cytoplasmic component that counters the damaging effect of ice crystals, does it?
Entropy,
Nonlin still arguing that anabolism is forbidden by the Laws of Thermodynamics, huh?
Allan Miller,
Yep.
Nonlin.org,
That’s cute. Dear Pazzy is a somewhat confused undergraduate at the University of Exeter. I expect you like him because, during his videos, you never react “that’s not right” — for the simple reason that he manages to confuse even himself. He even admits to having been flummoxed by the distinction between Permutations and Combinations.
On the other hand, when listening to a Professor of Mechanical Engineering at MIT, nonlin notices that the MIT gent is counting microstates wrong!
ROFL.
Here’s a Stanford Professor of Theoretical Physics. I’m sure you’ll disagree with him! <gggg>
[Actually, keiths and I are probably the only people who will agree with everything he says…]
DNA_Jock,
Oh yeah? Qualitatively, we have a contest between ‘missing info’, ‘order’, ‘unavailability of energy to do work’ and ‘energy spreading out’. I don’t have a particular beef with Stat Mech, but for chemistry, if we are comparing qualitative interpretations, the ‘order’ metaphor winds up particularly strained, and finds its mangled way into Every. Creationist critique. Evah. as an ‘ordering’ of components, largely dispensing with their energy altogether, a particularly rum state of affairs. “Look at a broken egg; imagine a film running backwards; shuffle a fresh deck”. That’s not Stat Mech’s fault, granted.
Missing info … well, I guess, especially given that photons are carriers of information. But there’s an unsatisfying lack of the mechanistic about that.
This made me chuckle though.
Σ P(i)=1 (ie 100%) where P(i) = N(i)/N for large N
I know at least one chap hereabouts that disputes this. It’s something you can’t say till you’ve checked, apparently!
I didn’t say you did. You asked me if I was sure their resistance was down to them being amphibians. If I had claimed that I would have been begging the question. There is much more complexity to be explained which simply being an amphibian does not address.
What amphibians have in common is that they are all exothermic. Most amphibians do not have the amazing ability of wood frogs because they don’t need it in the environment where they live. But no amphibian as free from the environmental fluctuations as birds and mammals. They are more tied to the local earthly conditions. But if they must follow external conditions then at least they can have the inbuilt wisdom to deal with the variations they have to experience. If they cannot isolate themselves from the environment in the way that birds and mammals do then they must live with the matching external and internal fluctuations.
In the words of Kate Bush,…. 🙂
No it doesn’t beat it because they are both consequences of the same thing.
Being more constrained by earthly conditions than say, the taiga vole these frogs must employ some ingenious adaptations to survive. Having more freedom, taiga voles can remain active all year round.
Both have very clever adaptations to deal with the situation in their own way.
Wood frogs can survive being frozen in up to seventy percent of their bodies multiple times during a winter. They divert the fluids in their bodies to areas where freezing will do less damage and their cells take in a large quantity of glucose (among other products) which acts as an antifreeze. This allows the cells to remain liquid while the extracellular fluid freezes. These creatures actively encourage freezing of this fluid by the use of ice-nucleating agents. Small changes in glucose levels are dangerous for us but wood frogs can cope with blood sugar levels one hundred times higher than normal.
The environment controls the frog’s temperature, but the voles attain more freedom from the earthly environment by insulating themselves with fur and large deposits of subcutaneous adipose tissue and by constant eating to replenish their glycogen levels.
And at the opposite extreme to the Arctic there are the frogs of the desert. There are a dozen or two species of frog that live in the arid deserts of Western Australia. How do they survive? They bury themselves in the sand for months at a time and form a cocoon type covering with layers of dead skin to prevent water loss. Only their nostrils remain open to allow breathing. This is just one example of the necessary adaptations they have come up with.
From Australian Geographic
Using standard evolutionary thinking, all these vital interdependent adaptations in each case will have been somehow ‘stumbled upon’ and ‘cobbled together’. How lucky is that!
Allan Miller,
LOL. I quite agree. And in almost all cases, the ‘dispersalist’ explanation works just fine, so long as one recognizes the effect of degrees of freedom on things like heat capacity. One of the things I liked about Prof. Susskind’s approach was how strongly he emphasized the primacy of entropy (and energy) over derived concepts, such as temperature.
Using entropy as a measure of ignorance helps to explain nonlin’s counting problem: he sees much lower entropy in small systems than physicists do, because nonlin knows things about the system that are not true (specifically, that the energy is always evenly spread amongst individual atoms). This ‘knowledge advantage’ of his leads to contraventions of the 2nd Law. In other news, it also means Arrhenius was wrong.
CharlieM,
No luckier than having some Intelligence that just kinda knows what conditions the frogs ‘must’ face, makes sure their genomes are poised to make the necessary changes without excessive disruption elsewhere, and then makes the changes, all by means mysterious. If we’re trading “why, dat’s crazy talk” lampoons, that is.
Aminoacids are not life as Miller-Urey sadly found out.
This is still incoherent gibberish, but marginally addressable unlike the rest of the comatose output. Let’s see… Not “now” – the entropy asymmetry is the main point of this study. No, everything in nature is NOT “undirected”. Only zombies “think” so.
You’re confused once again. The living do anabolism, The inert, as in abiogenesis BEFORE life does NOT do anabolism because it’s impossible. Because of entropy. Look it up… anabolism.
Meanwhile, you learned from me that you cannot dissipate 100% of energy and STILL end up with some local entropy decrease. Of course, your repair tech could have also told you that freezing 2 liters of water dissipates more than freezing just 1 liter.
BTW, you and your buddies also learned that entropy MUST be normalized.
And of course, you learned from me that sugar crystals are no more magical than other crystals (they do NOT form “spontaneously”), that crystals have lower entropy than their liquid state and that, of course, solution is NOT the liquid form of a crystal.
Just a little more and you will be able to call yourself a true biochemist…
Like understanding that life is PARTLY a lowering-entropy-machine which it does by accumulating chemical energy. That entropy-lowering is fundamentally different and incomparably more difficult than entropy-increasing. And that entropy-lowering from simple molecules to the cell is simply not possible as an “undirected process” on the account of entropy asymmetry.
Don’t take it personally. I disagree with anyone that is wrong, the pope included. Heck, I disagree with myself once proven wrong (that’s your mechanical rabbit cue). The guy need not be correct 100% to be “pretty good” and professors also can be wrong while “pretty good”.
Meanwhile, you learned from me that entropy needs normalizing, that melting ice is not a good source of energy and that biochemical energy is awesome and amazingly low entropy. You also learned that fluctuations are forbidden in thermal equilibrium despite the theory. But this has yet to sink in. So what will you learn next? See above your buddy being tutored.
Once again, you’re wrong. In fact, I write against the simple ‘order’ metaphor in par 1.
Current models of entropy assume the gas particles in a chamber to be independent (sometimes represented as pebbles on a Go board) and explains their never observed convergence on one side of the chamber as only due to that particular microstate having a very low probability(*).
As far as ‘broken egg’, there’s no metaphor there and no mystery. Broken egg has broken chemical bonds, hence demonstrably higher entropy. And it’s NEVER going back to a viable egg. Try to argue against that.
Only at equilibrium. I volunteer a general claim. All you need is ONE counterexample to disprove. That should be much easier, so where is it? Your lonely counterexample?
False. Prove it!
You should have looked at my answers, not at your comatose and incoherent gibberish.
I know. That’s what makes your declarations about “undirected” so funny and incoherent! Finally you’re getting it!
“study” 🤣
This is a very weird way for you to admit defeat, but I take it. How are you Ms. Zombie?
And thanks for the entertainment.
Heh heh. You think loads and loads and loads of abiogenesis research has been done. But all you ever mention is Miller-Urey. OK, you once mentioned Oparin, and have some bizarre notion that synthetic biochemistry is abiogenesis research. Other than that, you pound the Miller-Urey nail ad nauseam. That’s it. That’s the whole of abiogenesis research, which should have had a result by now, according to you. What larks!
Haha. I suspect you just looked up ‘anabolism’ for the first time after I used the term. Your arguments against abiogenesis are also arguments against anabolism, if you did but know it. You insist, with grotesque cluelessness, that coupled reactions cannot create a net negative ΔG. That means anabolism cannot happen. Look it up, as some blowhard might put it.
Whether local entropy decrease happens does not depend on the percentage of energy dissipated! 🤣🤣🤣🤣 All it requires is that some is dissipated.
I reckon the fridge works harder with the door open than with it closed, however many litres of water are in it. You don’t, it seems.
Your electricity bill is a fair proxy for the work that must be done to maintain the non-equilibrium state.
Leave honey in a cupboard with the lid on at constant temperature, you still get crystals. That’s spontaneous.
It’s not the molten form, but it’s definitely a liquid. And its entropic relation to the disassociated form is contextual. This is an important fact about entropy: it’s not intrinsic.
They aren’t. This is just a Litany Of Wrong, with added chest-beating.
It is quite a thing to behold, to see the Dunning-Kruger Ego in full flight. What spoils the fun is the realisation that such an individual will never see what an unholy hash they are making of things, but continue to strut chicken-necked amongst the wreckage.
You write against it, then fall victim to it. So you don’t even listen to yourself. Example follows shortly.
Hahahahaaaa! Broken chemical bonds when you crack an egg!
Hahahaaaaaa! Broken chemical bonds mean higher entropy!
Right answer, wrong reason.
Haha. No, I’m not going to argue against the ‘order’ metaphor. The thing you ‘write against’.
Exits, chuckling.
Talk about incoherence. Nonlin, you cannot declare, let alone in the same paragraph, that the living do anabolism and that anabolism is impossible because of entropy. If it was impossible, whether the problem was entropy or not, the living would not be doing it. Simple logic.
P.S. The results of the Miller-Urey experiment were complex chemicals built from simpler ones. That’s anabolism. No surprisingly, entropy didn’t mind.
That’s what you said that I was responding to. I quoted you.
That’s what I said.
And then that was your response.
So
a) I never said amino acids were alive.
b) It neatly disproves your specific claim that simple molecules cannot come together to form more complex molecules.
You know that b) is true which is why you responded to something I did not actually say, namely that I said they were alive. I never used the word life in fact. So it’s clear that you cannot have a actual discussion as you are unable to internalize what other people are actually saying, rather you twist what people say so you can respond with something that seems true to you.
So, sure, Miller-Urey never made life. I never said he did. I never said life was falling out of the sky.
I said complex molecules.
Therefore if we define Abiogenesis as “simple molecules come together to form complex molecules and cells” and we define amino acids as complex then Abiogenesis has been observed.
How’s that foot feeling?
What, precisely, is the claim that you are volunteering?
All I would need to show is a system that is not exchanging energy with the ‘outside’ world and experiences a decrease in entropy, per nonlin.
[Note: spontaneity don’t enter into it.]
If only someone practicing nonlin physics had offered up an example of such a system…oh, look:
Verily, a “Litany Of Wrong, with added chest-beating”.
After a while, it just gets awkward. Still somewhat amusing, mind you, but awkward.
Allan Miller,
The ‘100%’ thing is a bit of a smokescreen. I’m not sure when it suddenly became a condition for Lambert’s to be a valid qualitative descriptor.
Conventionally, dissipation is the bit left unavailable from an initial quantity of energy after work has been done by it. So it can’t be all of that energy if work is done. Nonetheless, in the example I gave, an upper reservoir connected to a water wheel, 100% of the potential energy in the reservoir is dissipated – ‘spread out’, in Lambert’s terms – when the reservoir is empty. It is unavailable for work at the reservoir. Extending the bounds of the system to include the outflow and water wheel, part of the dissipative process is intercepted – the ‘capacity to do work’ is utilised to actually do work with complete dissipation of the remainder. Alternatively, if a sluice is closed to cut the wheel out of the system, it reaches equilibrium with 100% of the converted energy being lost. Nonetheless, at the reservoir, the same process takes place either way. Energy ‘spreads out’.
If processes weren’t dissipative – if energy did not ‘spread out’ – they could not do work.