As discussed here extensively, nothing in “evolution” makes any sense: “natural selection, fitness, speciation, human evolution, gradualism, divergence of character, UCD, TOL, etc. etc.” Not one makes sense. Meanwhile, the “evolution” argument is just one big “affirms the consequent” logical fallacy, while Paley’s excellent argument has never been overturned, and an intuitive intelligent design detector can be used to easily disprove “evolution”. Is there a need for any more proofs? Not really. Are there any other proofs? You bet. Take entropy for instance…
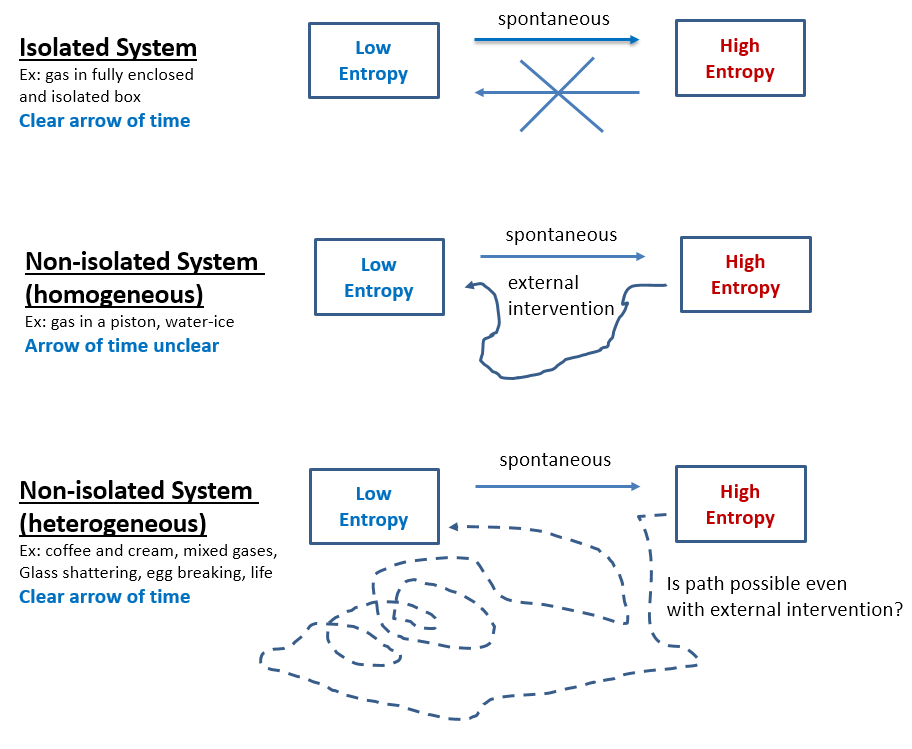
Figure 1
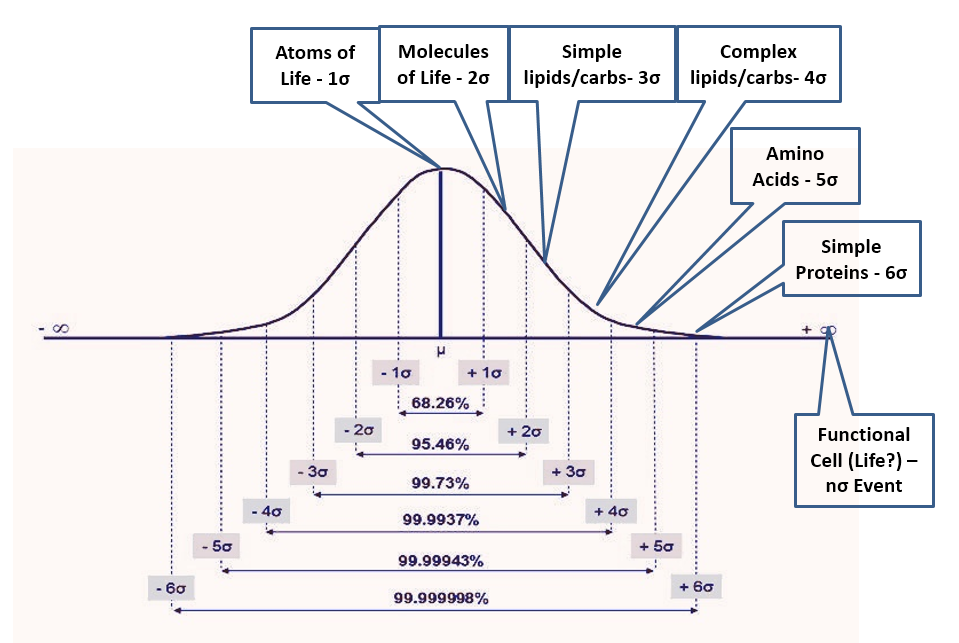
Figure 2
- Second Law of Thermodynamics shows that a spontaneous process cannot also revert spontaneously. This is because spontaneous processes always increase the system’s entropy. A uniform gas in a chamber will accumulate in a corner only with external intervention and spontaneous chemical reactions can only revert if external work energy is applied. Current models of entropy assume the gas particles in a chamber to be independent (sometimes represented as pebbles on a Go board) and explains their never observed convergence on one side of the chamber as only due to that particular microstate having a very low probability(*). However, gas particles always interact with each other (Brownian motion) while pebbles do not. Thus, a reliable way to know that entropy of a system increases is if work energy could be obtained when transitioning from the low to the high entropy state while energy is always required for the reverse process.
- Total entropy of an isolated system can never decrease. Entropy is currently assumed just a statistical law. Thus, if N molecules are in an isolated system (box), the number of microstates associated with j of them being in one half while N-j being in the other half is Ω = N! / (j!*(N-j)!). If N is small, fluctuations seem possible, but before N increases to anything measurable, the probability of fluctuations rapidly decreases to nil. Furthermore, even these theoretical fluctuations, as improbable as they are, might be impossible since the statistical view does not account for molecular interaction observed as Brownian motion and as gas resistance to compression and expansion. Better fundamentals or statistics, either way entropy will never decrease spontaneously in an observable system (Fig 1.a).
- Decreasing entropy is not the reverse process of entropy increasing. That is why a broken egg coming together is easily identified as unreal and a reversed movie of its real shattering. The known laws of physics are the same forward and backward (time-reversal invariance), therefore the reverse shattering process of an egg would not violate any law, but only because these laws are always idealized. Supposedly, if just the right forces are applied to the broken pieces, the egg will come together. In reality this is impossible, and not because the unbroken egg is a highly unlikely microstate, but because entropy increase is not directly reversible even in non-isolated systems. This irreversibility holds for all heterogeneous systems, including life which is perhaps the most heterogeneous system of all. Entropy increase is directly reversible only for homogeneous systems and only if in a defined space. For instance, an expanding gas in an ideal piston creates a force that, when reversed, compresses the gas back into its original state. However, a solid cube of ice can be easily melted by increasing the temperature, but the original ice cube will not reconstitute by lowering the temperature, hence this process too is irreversible despite the cube of ice being homogeneous (Fig 1.b). As far as heterogeneous systems, even separating two mixed gases is way different than the original mixing process, hence mixing is irreversible (Fig 1.c). Entropy decrease is not only different, but also much more complex than entropy increase which is usually spontaneous. Abiogenesis is the entropy-lowering reverse of the biologic decay process, and therefore – if at all feasible – much more complex than adding chemicals and energies.
- Once in equilibrium, a “primordial soup” does not change spontaneously. Life is metastable – it requires certain forms of energy to sustain and spontaneously decays when it no longer receives that energy as well as after the end of the normal lifespan of the organism. It was hypothesized that random fluctuations can spontaneously create compounds and structures given enough time. Abiogenesis, as a reverse-decay process, cannot simply be an outcome of Brownian motion of the chemicals mix because a perpetual motion machine powered by decay and abiogenesis cycles would violate the ‘conservation of energy’ principle. Experimentally, one can confirm that chemical blends in static equilibrium never transition spontaneously into a different equilibrium state (this includes oscillating reactions after the settlement period).
- A “primordial soup” cannot generate life even if energy is applied. It was hypothesized that abiogenesis can be a product of tidal pools, deep sea hydrothermal vents, and the undersurface of ice caps where persistent and abundant energy is available in the form of thermal and electrochemical gradients. Indeed, energy can throw systems off balance and create all kind of chemical compounds and physical structures. However, as the energy applied increases, a complexity limit and hence a dynamic equilibrium is reached where molecule destruction offsets their creation and, if even more energy is applied, molecule destruction dominates, eventually leaving the experimenter with gunk and none of the desired molecules. Miller–Urey and subsequent experiments were not ended because they reached their goal – life – nor because they ran out of energy and materials, but because they reached this dynamic equilibrium, and by adding more of anything would have left them with fewer of the targeted compounds. The amino acids obtained were not the end product but the intermediate between the original molecules and the useless gunk that was the product of the Maillard reaction caused by the energy applied to the system. More complex molecules (and maybe life itself one day) can be created by intelligent designers adding targeted compounds and energies. Then “why can’t natural processes somewhere somehow just mimic the intelligent designer in this vast and almost timeless universe?” The better question is: “why insist on natural processes when the model to be mimicked is that of the intelligent designer?”
- If natural processes were capable of generating life, the environment would be full of intermediate bio-compounds. Life is so complex that laboratories have no hope of replicating it in the foreseeable future. However, if abiogenesis were an outcome of natural processes, the cell structure would be produced only from subsystems and complex biomolecules that in turn would depend on simpler molecules down to H-C-O-N, the atoms of life. A “primordial soup” capable of generating life, thus must contain all intermediate compounds from the atoms of life to the most complex biomolecules and subsystems in an ever-decreasing ratio as complexity increases. Not knowing anything about how this process would work (or even if possible), the most reasonable assumption is a normal distribution of outcomes with life being an n-sigma event (with n unknown) while the availability of the atoms of life being a 1-sigma event and anything else falling in between (Fig 2). Many x-sigma events would be required for each (x+1)-sigma event, with a good first approximation given by the normal density function. Thus, the 2-sigma event could be the basic molecules of life (water, methane, etc.), and we would expect only one of these events for every seven of the 1-sigma events. This approximation would further yield (in one scenario) 1/7 fewer molecules of life than atoms of life, 1/17 fewer simple lipids and carbohydrates molecules (3-sigma) than of molecules of life, 1/43 fewer complex lipids and carbohydrates (4-sigma) than 3-sigma events, 1/110 fewer amino acids (5-sigma) than 4-sigma, 1/291 fewer simple proteins (6-sigma) than 5-sigma, 1/771 fewer complex proteins (7-sigma) than 6-sigma and then – rule of thumb – 1/1600 (8-sigma), 1/3800 (9), 1/9100 (10), 1/22k (11), 1/52k (12), 1/126k (13), etc. fewer of each additional sigma event than previous event where 8+sigma being (this scenario) nucleic acids, short chains, long chains, organelle subsystems, organelles, other critical cell components and finally the fully functional biologic cell – the n-sigma event which is not quite life but good enough for this analysis. Then how can we test this?
- Apart from life itself, the complex molecules of life are nowhere to be found in the universe. To test the ‘natural processes’ hypothesis of abiogenesis, one must observe the intermediate components of life in nature and in the ratios estimated above (or from another reasonable estimate). In addition, one must observe the spontaneous transitions (aided by energy) from simple to complex even if not all transitions are observed at once. Earth is “polluted” with life down to the deepest ocean trenches, therefore the first focus is the extraterrestrial space where, too bad, the largest confirmed interstellar molecules have a maximum of 13 atoms (apart from C60/C70 fullerene). Back on earth we see all intermediate components, but only within life itself. Outside of the cells, aside from the simplest biomolecules, we only see products of decomposition that are never in the ratios associated with abiogenesis, meaning we never see increasing molecule complexity in decreasing ratios resembling anything reasonably expected. Abiogenesis is not happening due to the irreversibility of the entropy increase and for the same reason egg breaking, butter melting, gas mixing, etc. are not reversible processes. Humans can only create a few of the complex molecules, although most always aided by life itself, and even then the power of synthetic biology is severely restricted. The more complex, the harder these molecules are to obtain and the faster they decay instead of spontaneously combining with one another to form even more complex compounds and ultimately life.
- Miller–Urey style abiogenesis experiments are ill conceived, hence doomed from the beginning. To be more specific, they are only good for PR (public relations) given the irrelevant “organic compounds” created that raise the hopes of the believers. Trying to obtain an automobile from scratch by mixing chemicals and energy, qualifies the person attempting as delusional and the one selling such vision as charlatan. So why would those attempting the same with life – which is infinitely more complex than an automobile – not also be labeled charlatans and delusional? Abiogenesis experiments belong to the Reverse Engineering category of processes and, when done right, they are very different than Miller–Urey. Their starting point is never some “primordial soup”, but the most advanced compounds available, preferably already organized in working subsystems. Swapping organelles or parts within organelles, exposing organisms to various environments, attempting to revive dead organisms, substituting engineered subsystems and so on are part of the hard work with long tradition and already being done in medicine and many industries for other purposes than to prove abiogenesis. If and when someone will be able to reverse the decaying and dying processes, we will know that abiogenesis is possible as an act of Intelligent Design creation. To confirm abiogenesis as an “unguided process” we would have to observe reverse-decay and reverse-dying processes happening in nature, not in a lab. Yet 2nd law proves this impossible.
- Is abiogenesis not feasible because it was a unique event? If true, abiogenesis would be a “materialistic miracle” and furthermore not just one, but a long series of “materialistic miracles” since a long series of – so far unknown – events are needed to get from atoms to the simplest organism. Yet one of the tenets of materialism is “no miracles” showing the inconsistency of the materialistic “unique event” assertion. And of course, physics and chemistry transformations are never unique. And even if entropy allowed for abiogenesis, the laws of life do not follow from any priors (physics, chemistry, mathematics). Life has a drive to survive and leave off-springs which entails harm avoidance, immune system, metabolism, food seeking, homeostasis, growth, reproduction, and body structure. Without these, any cell would start decaying the instant it was formed as in fact it does as soon as it no longer is alive. Despite having lasted almost since the formation of The Earth, life is metastable – one knock and it dies and then decays. This is unlike other negative entropy machines that can be restored (rebuilding proportional with the damage).
- Other considerations.
- “Dissipation-driven adaptation of matter” (J. England, MIT) claims that life is inevitable because life “absorbs and dissipates more energy from external sources” leading to faster entropy increase. However, there is no law that entropy has to increase faster. In addition, most of the entropy in the universe is captured by black holes with life having a nil contribution to that entropy.
- Some claim they have obtained “protocells” that seem to mimic real cells at least in part. However, “protocells” are to biological cells as fool’s gold is to real gold.
- “Kolmogorov complexity is lowest at low and high entropy and high in the middle hence life is supposedly inevitable (S. Carroll)”. However, life is not complexity. Life is much more than snowflakes, vortices and chemical reactions (candle burning). And most certainly, life is not the complex swirls of cream mixing into coffee on a journey from low entropy to high entropy (both having low complexity). In addition, unless very specific external action continues to be applied to maintain those patterns, they soon disappear like in sand dunes exposed to shifting winds. The patterns therefore do no “arise”, but are created by an external force.
- “Gradients of energy in deep vents are responsible for abiogenesis”. But all organisms from these exotic places are very similar to any other ones found elsewhere, hence all likely have the same origin. In addition, no free floating organic compounds (aside from decay byproducts) have been found there to suggest ongoing abiogenesis. And, aside from the simplest molecules, no spontaneous transitions from x-sigma to (x+1)-sigma bio complexity has ever been observed around these deep vents either.
- Of course life does not violate 2nd Organisms do conform to 2nd law when they decay as soon as they die. In addition, as observed by Erwin Schrödinger, “the increase in entropy from turning our low-entropy food into our high-entropy waste is greater than the local decrease in entropy from making the well-ordered structures within our bodies”. Nothing special so far – a refrigerator does the same: creates a zone of low-entropy while the entropy of the whole system increases and for as long as it’s fed energy.
- Randomness can theoretically account for any bizarre occurrences including Paley’s watch and F. Hoyle’s 747 in baby steps if enough time is given. But no such event was ever observed. In addition, breaking down the unattainable complex system into a combination of simpler components, each with higher probability of occurrence makes it no easier as the probabilities of all subsystem have to be multiplied to get back to the complex final assembly.
- Some claim that life itself prevents abiogenesis by ingesting all intermediate molecules spontaneously formed, but this can be easily prevented in sterile labs. In addition, all complex intermediate molecules observed outside of cells are due to decomposition, not abiogenesis.
- “Evolution” corollary number 1. If abiogenesis is impossible as an undirected, natural process, then whoever is responsible for abiogenesis is also responsible for the biologic landscape past and present, therefore “evolution” is also impossible as an undirected, natural process.
- “Evolution” corollary number 2. It is easy to verify that nothing ever “evolves” in the nonliving nature. Life is said to be “just chemistry”. These two combine to: nothing “evolves” in the living either. Solar systems, geographical features, fluid eddies, chemistry, snow flakes, etc. all go through their life cycles, and all are different from each other, but the life cycles of the newer entities are no more “evolved” than the life cycles of the ancient ones.
- “Evolution” corollary number 3. Presumably, “evolution” has not ended. And if ongoing, then one must see the normal distribution of the different transitioning organisms (the intermediary), just as we would see if abiogenesis were true. If humans evolved from monkeys and “evolution” is ongoing, then humans must still be in transition especially since the human population is one of the largest of all mammals and, the more individuals, the more “evolving” opportunities. The older Darwinists replied with a hierarchy of races. But that reply is not only fashionably repugnant, but also false and, amazingly, contrary to [at least] the Abrahamic religions that have always known better.
- In conclusion, abiogenesis is nothing more than the decay process running backwards, therefore easily visualized, yet impossible according to the second law of thermodynamics. In other words, “evolution” is nothing more than imagination run wild. Expecting abiogenesis to be within reach if only the proper forces and chemical compounds were added is as wrong as expecting the broken egg to come back together if only the proper sequence of forces were applied to the broken pieces.
Summary:
- A spontaneous process cannot revert spontaneously.
- Mixtures will never ever spontaneously separate per second law.
- Decreasing entropy is not the reverse process of entropy increasing and also much more complex.
- Once in equilibrium, a “primordial soup” does not change spontaneously.
- A “primordial soup” cannot generate life even if energy is applied due to dynamic equilibrium.
- If natural processes were capable of generating life, the environment would be full of intermediate bio-compounds.
- Apart from life itself, the complex molecules of life are nowhere to be found in the universe.
- Abiogenesis experiments belong to the Reverse Engineering category of processes.
- Miller–Urey style abiogenesis experiments are ill conceived, hence doomed from the beginning.
- Abiogenesis unique event conflicts with the “no miracles” clause of materialism.
- Even if entropy allowed abiogenesis, the laws of life do not follow from any priors (physics, chemistry, mathematics).
- “Evolution” corollary number 1 – no abiogenesis, no “evolution”.
- “Evolution” corollary number 2 – no “evolution” in the inert and “life just chemistry”, then no “evolution” in the living.
- “Evolution” corollary number 3 – no intermediate “evolving” entities, no “evolution”.
- Being a decay process running backwards, abiogenesis is as impossible as a broken egg being reconstituted by the “proper sequence of forces”. “Evolution” is also nothing more than imagination run wild.
(*)R. Penrose “The Emperor’s new mind”; PBS SpaceTime “The Misunderstood Nature of Entropy”; Sean Carroll “From Eternity to Here”, etc.
Links:
Abiogenesis: The Faith and the Facts
James Tour: The Mystery of the Origin of Life
Chirality, Maillard – caramelization, characterize the structure at every step:
https://creation.com/why-the-miller-urey-research-argues-against-abiogenesis
https://evolutionnews.org/2014/06/squeezing_the_l/
https://www.ncbi.nlm.nih.gov/pubmed/21422282
Entropy of a box of molecules
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2253472/ https://en.wikipedia.org/wiki/Normal_distribution#Cumulative_distribution_function
http://physics.bu.edu/~redner/211-sp06/class-engines/class25_secondlaw.html
https://www.quora.com/How-quickly-is-the-entropy-of-the-sun-changing
https://www.thoughtco.com/how-many-atoms-in-human-cell-603882
https://www.amazon.com/Mysteries-Modern-Physics-Sean-Carroll/dp/1598038699
https://en.wikipedia.org/wiki/File:Elements_abundance-bars.svg – abundance in the solar system
https://en.wikipedia.org/wiki/Miller%E2%80%93Urey_experiment
https://en.wikipedia.org/wiki/Proteinogenic_amino_acid
https://en.wikipedia.org/wiki/Artificial_gene_synthesis
https://www.scientificamerican.com/article/a-new-physics-theory-of-life/
https://en.wikipedia.org/wiki/Dissipative_system
https://pubs.usgs.gov/gip/dynamic/exploring.html

You posit a “replicator” and “precursor” for which you have NO BASIS. How about you come back to earth?
And yet they are, as you complete a cycle. The universe aside, one is increasing entropy locally and it’s TRIVIAL. The other decreases entropy locally and it’s HARD. QED.
I can and MUST when there’s not ONE plausible alternative.
The point was “way easier”. Once again, for every process I give you 100% examples of decreasing entropy being harder than its reverse entropy increase. All you have to do is come back with ONE counterexample. ONE. If not, cut the crap.
Yeah, I do need this “lesson”. Now explain what that nonsense means… or cut the crap.
Not really. Just making your life easier. Obviously from cell to you it’s another IMPOSSIBLE road. We know that because entropy also forbids “evolution” as explained.
“There is no cell on earth that was composed”. Period. But if you are to make a cell – as we know cells to be – then you better begin with the most basic ingredients of the cell. Learn from Miller-Urey. They were overjoyed because of amino acids and have been peddling that crap ever since. Not because of smelting some iron, mind you.
Nonsense. Where is this system called “locally” where entropy decreases despite ALL (100%) of the energy being dissipated elsewhere?
Forget the whole of abiogenesis. We don’t even see the steps towards that abiogenesis – Fig 2. And that’s just the confirmation of my main thesis: entropy decrease is hard in simple systems and impossible in biology – Fig 1. Unlike entropy increase which is trivial.
Let’s explore, shall we? Why is this particular experiment failing? And when always failing without ONE clue, the most reasonable conclusion is “not possible”. Logic 101. Furthermore, as I show, entropy decrease is devilishly hard, so failure is TO BE EXPECTED.
“Hard” without a hint of a reason? Not possible. There’s nothing “hard” about sampling some chemical composition.
FALSE. Forget bullshit abiogenesis and “evolution”. We have an entire field of synthetic biochemistry and billions of people are desperate for results. Yet said field only amounts to bacteria farming. You know, like cowboys for bacteria. So the damning evidence is right there.
Chose one:
1. they were trying to prove abiogenesis
2. they were trying to DIS-prove abiogenesis
3. they were building a model railroad
Allan Miller,
Too small snippets and missing links to understand what’s being said. Pick and choose is not the way to go.
Entropy,
Who said monkeys aren’t funny?
Allan Miller,
Recanting? Won’t hold that against you.
Do you even understand how nonsensical this claim is? You’re saying that A-to-B is the reverse of B-to-A, regardless of the intermediary steps being different, if abiogenesis is true. But they’re not reverse paths of each other if abiogenesis is false. So, if scientists figure out how abiogenesis happened, then everything we see around will magically be defined as reverse-paths, regardless of intermediary steps, just because abiogenesis is true!
Your desperation is showing Nonlin. I never dreamed you could be that nonsensical. You’ve been a champion of nonsense, but this is well beyond anything I’ve read before.
Great! Then entropy is not a problem for abiogenesis. Thanks for playing.
You certainly are.
Holy crap! Then how is it possible for your body to keep itself alive Nonlin if entropy decrease is impossible in biology?! How could you even be born at all? How could there be any life at all!?
Holy holy holy holy holy holy holy holy crap!!
nonlin writes:
Oops, nonlin, you clipped off the first half of my sentence. What I actually wrote was:
Do you disagree? Are you actually claiming that the entropy of an isolated system can decrease? You appear to be denying the 2nd Law and, by your selective quoting, you appear to be trying to hide your error. You are plumbing new depths of incoherence: it was your example of a 3quanta-4particle system combining with a 2quanta-1particle system, remember?
And, according to nonlin-physics, the entropy in this isolated system goes from Kb.log(4) to zero. So, safe to say, nonlin-physics is wrong.
It’s truly amazing that Nonlin thinks that she can clip-quote and we will forget the context of our own answers, just because she forgets context after reading one sentence.
I’m not going to say that her practice of selective clipping is dishonest though.
That would be ‘recognising and pointing out an error one has made’. I do endeavour to be intellectually honest. I realise this is an alien concept to you.
This is exactly your own modus operandi, except that you collate multiple responders within the one post. When you do it, I put in the effort to scroll back to establish context. It’s not difficult.
In order to investigate a proposition, it is necessary to ‘posit’ that thing being proposed. This is basic stuff. ‘Discuss abiogenesis without taking the stance that it occurred’. Good one.
Haha. ‘The universe aside’. Yes, let’s ignore that these are open systems.
One is thermodynamically spontaneous (negative ΔG), the other isn’t (positive ΔG). Nonetheless, neither path violates the 2nd Law of Thermodynamics. A-B and B-A (by a different path) don’t require a statistical fluctuation. One requires energy, the other yields it. Energy is freely available, so that’s certainly not the problem, your misunderstanding of ‘equilibria’ notwithstanding. And actually, both are ‘easy’. Splitting water by photolysis is a doddle. Trivial, one might say.
Logic fail.
It’s not a question of how easy you find it! 🤣 Good grief. Crystals form spontaneously in the right conditions. You don’t need to do work to make this happen; it has negative ΔG. Once formed, they are in a higher-entropy state. You have to do work to break this state. It is not spontaneous. Even if it is ‘easy’, cos you don’t have to fiddle about so much 🤣
Nonlin-entropy forbids all kinds of possible things. Actual entropy, not so much.
I’m not questioning that the elements must be present. But you are making a stronger claim regarding stoichiometry, effectively – that the proportions in the pre-abiogenesis state must reflect those in the post-. There is also the question of cofactors, catalysts, physical substrates etc, to consider. No other ‘manufacturing’ process has such tight specifications! Nothing beyond that which is present in the final product can be considered. And therefore manufacture is the precise opposite of deterioration. Sure.
It’s cute – the only work Creationists have ever heard of is Miller-Urey. Hammer that nail, nonlin!
Consider a water wheel between a reservoir and a pool. When all the water is in the upper reservoir, all its potential energy resides there too. It starts to flow, giving kinetic energy. It hits the wheel, turns it and hence the wheel gains the capacity to do work – local entropy decrease. But 100% of the energy in the upper pool has dissipated, as has the kinetic energy in the stream, even though some of it has done work to lower local entropy at the wheel.
I have already conceded, several times, that abiogenesis may not even be possible. But the reason for that is not your addled view of entropy.
There is no need to provide a reason. Logic 101. It is logically possible for things to be true but be beyond the approaches so far tried to replicate. One does not have to say ‘why’ for this to be valid. Indeed, knowing why would imply some knowledge of the underlying truth, rendering the demand for ‘reasons’ a paradoxical one.
If that state is below the limit of detection of your equipment, then of course it’s ‘hard’.
It shows a particularly naive view if you think the conditions in synthetic biochemistry would be guaranteed to produce life if it were possible, and that this failure ‘disproves’ possibility.
You need to dig out your notes from Logic 101 – the only course you ever took, by the looks of it, and I doubt you passed. Thumb your way to ‘proving the negative’.
None of the above.
Mind you, here we are along with all the rest of diverse life on Earth. Our planet had a magma “ocean” during the accretion phase and prior to the condensation of liquid water. Had to be sterile and void of carbon-based life then. Other than an abiogenesis event or panspermia, what hypothesis fits the facts.
Alan Fox to Allan Miller,
I agree with you. However, I’ve been writing “if there’s a problem for abiogenesis, it’s not entropy” to try and keep Nonlin’s focus. I think that has been Allan’s intent as well. However, it seems like Nonlin and “keeping focus” are antithetical. Nonlin is simply unable to distinguish between “abiogenesis happened” and “if there’s any problem with abiogenesis, it’s not entropy.”
But it’s HARD and ignoring the context is TRIVIAL! Keeping context is so rare that it can be precious. QED.
🤣
Indeed.
“Thou hypocrite, first cast out the beam out of thine own eye”
Mathew 7:5
Since it would be found among the myriads of compounds made by microorganisms it would not just be hard, but pretty much impossible to detect and/or tell apart.
Entropy,
Entropy, why are you forbidding abiogenesis and evolution?
I see too much nonsense to address it individually.
So let’s recap… once again:
1. Entropy increase is spontaneous while entropy decrease requires intervention. And just because some crystals are common, it doesn’t mean abiogenesis is compatible with entropy. Those crystals are still less common than their higher entropy states. That includes salt which was so valuable that the word “salary” is derive from it and ice that you have to buy from store or employ a complex machine to produce.
2. The more heterogeneous the low entropy system is, the harder it is to obtain. And that is WITH willful intervention – forget about “natural forces”. Whoever thinks [ice] crystals have anything to do with abiogenesis is delusional.
3. If abiogenesis were possible, it would be the reverse process of biologic decay. Atoms-to-cell would be cell-to-atoms in reverse. One is entropy increasing, the other entropy decreasing. We see cell-to-atoms (or to simpler molecules) all the time and some think: “sure, why not the reverse”. But… see 1… one is easy while the other very hard. Whoever thinks there’s an alternative abiogenesis path must produce a modicum of evidence. Whoever thinks there’s an alternative to the cell AS WE KNOW IT (“primordial cell”), must produce that evidence.
4. Experimental evidence supports “abiogenesis is impossible” This includes direct abiogenesis experiments, natural evidence, and the severe limitations of synthetic biochemistry that is reduced to bacteria farming. This is expected given what we know about entropy.
5. Experimental attempts at biogenesis have all been of the reverse-decay type. This shows no other path to abiogenesis is even imagined (sci-fi aside). Entropy strikes again.
6. Life sustaining does NOT support the theory of abiogenesis. Only alive cells divide and grow. Dead cells DO NOT, and instead decay as expected from the unidirectionality of entropy. Abiogenesis must start from the inert and fails at every single step. For instance, we can jump to protein formation and see that said step of abiogenesis fails independently of before or after steps. And of course, there cannot be any abiogenesis without proteins. And of course, we see all the time the reverse of this step, aka protein decay.
6. Before claiming “current life prevents (eats) abiogenesis”, one must prove that something is produced and eaten. But nothing is produced in nature or sterilized labs. Furthermore, simpler compounds would have to be generated in huge quantities (Fig 2), so “too few to measure” doesn’t make sense.
7. The universe is almost always in some sort of equilibrium. Abiogenesis requires a VERY long chain of unique transformations. But the universe is almost always in some sort of equilibrium (as % of space-time). Static equilibrium does not allow for fluctuations, so no local entropy decreases there. Quasi-static equilibrium and dynamic equilibrium produce more of the same all the time, like lower entropy crystal growth or higher entropy gunk if too much energy is applied as in Miller-Urey. Disruptive transformations as required by abiogenesis are rare in the universe (volcanoes, supernovae, collisions, etc.) and many of those are generated by life itself (as in the cycles of life) which obviously could not have contributed to abiogenesis. And new biochemical compounds are inexistent outside of life produced ones.
8. Did I miss any objections to “entropy forbids abiogenesis”? Is anything above NOT related to ‘entropy’ and ‘forbids’ and ‘abiogenesis’?
One more thing, par 12 asks: if abiogenesis were true even if MIA, what other entropy-decreasing process in nature come closer? Is there a close runner-upper we can actually confirm experimentally to gain some confidence in abiogenesis-like events?
And one more, par 9: “And even if entropy allowed for abiogenesis, the laws of life do not follow from any priors (physics, chemistry, mathematics)”. So what good is a shiny new abio-generated cell other than just to observe its immediate decay?
This is particularly stupid. No, 100% of the energy could NOT have dissipated given that some of that can be harvested to lower local entropy somewhere. You can actually TEST this with your refrigerator: heat dissipation when cooling vs when door open. Cooling dissipates more!
I later pointed out that’s not an isolated system. Probably went over your head. Whoosh.
This might be another epic fail. What crystal do you know to form spontaneously? In an isolated system? Because else that wouldn’t be “spontaneous”. And are you saying some crystals are higher entropy then their liquid form? Which one?!?
Nonlin.org,
Hum. Besides it was already addressed, that’s too much nonsense to address, again, individually, so let’s recap:
Since local entropy decreases are an everyday experience, if there’s any problems with abiogenesis, entropy is not one of them.
QED
My favourite: “Though thou shouldest bray a fool in a mortar among wheat with a pestle, yet will not his foolishness depart from him.” Proverbs 27:22.
Indeed. My expectation is that (if it happened, of course), it would be in ‘dirty chemistry’, not the cleaner model systems of laboratory work. And what one would be looking for is perhaps just one or a few minute instances, not the Godzilla-out-the-swamp of nonlin’s fevered imagination. It would be like trying to detect a single bacterium in many litres of grubby medium.
Nonlin knows no middle ground. For her it’s either impossible or we should be swamped in abiogenetic gooey. Don’t you dare of thinking possible but scarce.
It has indeed dissipated. It is not where it was. ‘Somewhere’ is still Elsewhere. This is the essence of entropy change.
Both when the door is open and when it is closed, energy is dissipating. That’s why you need to keep it switched on. Playing with the rate does not change this.
I’m sure you’ll get over it.
Isolated system? Why that restriction? Crystals form spontaneously, given the right conditions. Let’s do some science! Create a saturated salt solution, dip a cotton thread in it and leave it a week. Crystals. Lots of ’em.
I think you are falling into the classic trap of over-applying the ‘entropy/order’ metaphor. Crystals are ‘ordered’, but entropy increases as they form. Which is why they can be observed to ‘just do so’, without you needing to do any work (in the physical sense: you don’t need to ‘apply’ energy). There is more capacity to do work in the pre-crystallised solution than there is when the crystal has formed. If you want to break it, on the other hand, you have to do work.
You’ve given me an idea which I must patent:
The osmotic improbability drive!
Allan Miller,
And not knowing what you’re looking for.
As it happens, there are four locations in the Ébrié lagoon where abiogenesis occurs on a fairly regular basis. The mud composition is just right, and the PNA replicators typically replicate once every twelve hours (tidal cycle). They can get quite large, up to a thousand molecules after five days — the record is nearly a million molecules, before a prawn comes by and eats it.
The tricky bit is finding one of these four locations (each about a millimetre across) in a 500 square kilometre lagoon.
And yeah, I made that up.
Returning to the serious business of 2LoT, apparently
does not constitute an isolated system, because nonlin says it doesn’t. I’m not seeing it exchange energy or material with anything else. I already explained that the 1 particle could have kinetic energy. nonlin thinks that because they don’t have to stick when they collide, that means they cannot. It’s truly ludicrous, but necessary to defend nonlin’s ‘preposterous and unique’ microstate-counting technique.
In open systems, spontaneous local entropy decrease can occur by simple coupling to wider entropy increase. Systems are not homogeneous; the spatial distribution of entropy matters.
To some extent, perhaps. Which would go some way to explaining why it is not a daily occurrence. But there is ground between ‘too complex to be common’ and ‘too complex to be possible’.
No it wouldn’t. Biological decay is caused mainly by other organisms – bacteria, if nothing else makes a meal of it. The reverse process would be bacteria stitching together bodies. I don’t see why anyone should accept that this is what abiogenesis must involve, however often you repeat it. It is fundamentally dumb.
See ‘proving the negative’. Failure to do something does not prove it impossible. Especially if the experiments weren’t even trying to do it. The only experiment you even seem to have heard of is Miller-Urey, indeed, which hardly seems consistent with the idea that there has been an exhaustive search of possibilities.
Citation please.
Life sustaining at ocean vents demonstrates that they are not in thermodynamic equilibrium.
No, ‘one’ mustn’t. That is an absurd demand. It is perfectly reasonable to postulate an ‘IF’ conditional, without the need to prove it true before one is permitted to examine reasonable scenarios contingent on that assumed truth. Doing this is the essence of science, indeed.
No, it isn’t. Every twinkle of light in the sky is due to non-equilibrium phenomena. If they were at equilibrium, no energy (light) would be escaping. More to the point, the earth is not in ‘some sort of equilibrium’. Your confusion of thermodynamic and other kinds of equilibrium is beyond correction, it seems, and quite the densest idea you peddle, among some stiff competition.
I think the densest is that abiogenesis could only proceed by the reverse of “biologic decay.” The absurdity is astounding! According to Nonlin an abiotic system would have saprophytes, some mixture of bacteria and fungi, ready to try and do their stuff in reverse. Who can make sense of such a thing? And this absurdity is reinforced by claiming that experiments about abiogenesis are of the reverse-decay type. I’ve never seen any experiment about abiogenesis involving putting saprophytes there hoping to see them doing their stuff in reverse, like vomiting a zebra. I kinda suspect that scientists would know that these saprophytes are life forms, and thus not possibly part of an abiotic environment.
“Since the universe is mathematical, every little monkey is a math genius”.
Yes, because no one has invented any tracing mechanism yet. Oh, wait. And why do any experiments when they could falsify your magical, convenient theory?
Aha. Another one of those cheap bullshit theories like “alleles add up to 100% of something something”.
What an incoherent nonsense. At least it adds up to 100% of something something.
I thought it might be a stupid clause like yours. Of course without the “isolated system” restriction, absolutely EVERYTHING would be “spontaneous”.
But then again…:
spon·ta·ne·ous
/spänˈtānēəs/
Learn to pronounce
See definitions in:
All
Medicine
Biology
adjective
performed or occurring as a result of a sudden inner impulse or inclination and without premeditation or external stimulus.
So no example?!? Did I get that right, you do NOT have ONE single example? Of course not. And I asked about “their liquid form” and you replied with “solution” which is awfully ignorant.
Tell you what: you’re not seeing anything because you haven’t done the experiment. Stop bitching, go to your lab, do the experiment, and report back at once. Just make sure you count them energy quanta and make sure none is broken or damaged. Now, make sure you have enough melting ice in the tank and begone.
Nonsense. As explained.
Let’s explore this. Possible or impossible? Separate cream from coffee once mixed? Repair a broken egg? Restore omelet into a viable egg? And where would abiogenesis fall on this scale? It would be off the chart!
Now, how easy are the reverse processes of all these? VERY!
Usually yes. But what about fossils? These guys hope to extract aminoacids (if not just contamination of course): https://theplosblog.plos.org/2019/04/ancient-amino-acids-from-100-million-year-old-dinosaur-feathers-in-amber/
Miller-Urey gave you aminoacids, so reverse-decay from there to cell?
It’s not “proving” but “supports”. Cool?
Besides Miller–Urey, there’s those guys with their vesicles (that btw don’t look anything like the cell walls), there’s synthetic biology trying to create proteins and failing (even if not specifically abiogenesis targeted), and more listed here: https://en.wikipedia.org/wiki/Abiogenesis , all basically trying to reverse some cell decay (and all trying to lower entropy). And of course we don’t hear from any failures – that’s just SOP.
What did I write and what did you reply? Unrelated.
The time has come to either prove or withdraw the hypothesis. It is NOT science to continue pushing a hypothesis while not making any effort to verify it.
You’re just denying the well established concepts of quasi-equilibrium and dynamic equilibrium. Who cares?
Just look at yourself. A true math “genius” who cannot even read.
I’m not very surprised that you had no answer to my point and preferred to make fun of yourself. That’s because I’m right, as always.
Had you attempted to follow the link and read, mabe you’d have saved this embarrassing comment. Wait, no, you would not have saved the embarrassment, for one, you cannot follow links, for another, had you managed to follow that one, by accidentally clicking on it, you would have missed the whole thing anyway because you cannot hold a thought beyond one sentence, and not very well.
Thanks for the entertainment. You truly make a spectacle of yourself. In the most ridiculous way. Amazing.
Entropy,
Actually, you’re right! If the attainment of a particular biological state is the ‘reverse’ of its decay, and that reversal has the reverse free energy change, simply changing the sign of the net ΔG, nonlin has successfully argued himself out of existence.
So people have both done enough experiments to confirm that abiogenesis is impossible, and aren’t doing any? Take your pick.
Either you don’t know what an allele is, or you don’t know what 100% means. Take your pick.
Ah, the No-Speakee-English defence. Either way, you have to power your fridge, door open or closed. Because energy disperses on entropy change; you have to replace it if you want it to keep going.
Entropy is definitely increasing universally, so in terms of overall free energy change, everything that happens kind of is… Of course ‘spontaneous’ in chemical thermodynamics relates specifically to ΔG, and whether it is positive or negative. There are many reactions in which it is positive in isolation but negative overall when coupled to another reaction. Thus the coupled reaction is ‘spontaneous’. That’s how you keep going. If your biochemistry was restricted to uncoupled negative-ΔG reactions, you’d rapidly grind to a halt. Non-spontaneous reactions are driven ‘forward’ by such coupling – which is not restricted to biological scenarios.
But back to crystallisation. Crystallisation releases heat. Exothermic changes are typically (though not universally) spontaneous. The energy disperses, resulting in a higher-entropy state. The spontaneity here is directly observable, because there is no activation energy.
Oh no! Science defeated with a dictionary! How will we ever recover? I once bought my wife flowers. Because … y’know, I’m spontaneous like that.
So solutions are not liquid? Anyway, my answer is all of them. How many crystals can you identify that don’t form unless you put energy in?
Hahaaa! Self-reflection really isn’t your strong point. Why don’t you stop bitching and publish your stunning findings somewhere with a little more prestige? I’d open with ‘abiogenesis is decay in reverse’. Follow up with a wide-ranging consideration of the many forms of ‘equilibrium’, then a definition of ‘spontaneous’ that has nothing to do with reaction kinetics. That will show them they’re not dealing with some ill-informed crank. 🤣
Nope. Absolutely true. As explained.
You are seduced by the ‘order’ metaphor, and visions of films run backwards. Thermodynamics is fundamentally about energy, not rearrangements of matter.
Nah. You’d need to know what the free energy change was. It is not (necessarily) the same as the free energy change of decay. There are a great many examples of systems in which the free energy of decay is not the free energy of synthesis with sign reversed. Including your own.
No. Reverse-decay is your idea, not mine. I think it’s bullshit.
No. I’m actually doubtful that a single experiment has been done which was expected to yield life.
Failing? It would be unusual for negative results to be published, or widespread research endeavours to grow out of such failures in sufficient number to ‘support the negative’.
But you just said … oh, never mind.
But you just said … oh, never mind.
I’m denying that the things you term ‘dynamic’ or ‘quasi’ equilibrium are in thermodynamic equilibrium – apart from a reversible reaction, which you initially declared FALSE. And I’m also pointing out your foolish claim that equilibrium forbids fluctuation. Not that I’m relying on uncompensated thermodynamic reversals, but you’re still wrong.
As explained ad nauseam.
P.S. Though delta-G would be too advanced for Nonlin.
Meantime nonlin will be powering his Christmas lights from the enormous energy release of scrambled eggs.
Allan Miller,
Well, I do think evolutionist-materialists do need some explanation for why, if life is nothing more than chemical arrangements in a bag, what prevents science from simply freezing a living human, for say a year, or even just a week, or three days, and then unfreezing them, all the same chemicals that made up that human still exist-right exactly where they were, so why shouldn’t they just live again as normal?
That is a different question from ‘entropy forbids abiogenesis’. Is there anyone in the broad ‘faith community’ who understands that thermodynamics, and indeed Life itself in the physical sense, is not solely about arrangements of matter? Can I get an Amen?
phoodoo,
Also (been thinking while I gave the lawn a quick late-season mow) how does ‘non-materialism’ deal with this question? Do you suppose a life-force beyond mere material interaction that is somehow squeezed out on freezing?
I mean, my simple answer is that freezing kills you.
More precisely, an “immaterial” living force is squeezed out by the “material” action of freezing. Who knew?
Science can’t answer why questions. Why is there a universe? Why are we here? why is the sky blue? We can give an explanation as to how the sky looks the way it does to us, which is fascinating, but why is unanswerable.
Ice crystals. Ice crystals disrupt the delicate molecular structures which are in aqueous medium.
But we are storing seeds.
https://en.wikipedia.org/wiki/Svalbard_Global_Seed_Vault
PS I might wonder why we would want to be frozen and resurrected. I’m quite comfortable myself with the idea of disappearing into the cosmos though not today.
No, it wasn’t a question of the philosophical why. It was what prevents it physically. If you think it’s simply because water crystallizes, I am skeptical. I think it’s because we have no what life is.
Allan said life is more than just physical matter. I have no idea what he means by that.
Had you included a link I could be sure what you are referring to but living organisms are more than just matter. They have to use energy to maintain themselves out of equilibrium with their environment.
Allan meant that the energetic relations of the matter are every bit as important as their physical composition and arrangement. They are much harder to ‘freeze’. Creationists seem frequently to think of atoms as inert building blocks that can simply be shoved into place (somehow). This informs their cartoon view of the 2nd Law of Thermodynamics, among other misunderstandings.
When you freeze a body, there is no means of suspending the activity of that body across its entirety simultaneously. You’d have to get it to absolute zero instantaneously, then return it to normal temperature instantaneously. Any slower process disrupts interaction during its progress.
That said, freezing can be tolerated by some species – wood frogs survive freezing happily. But not humans; it kills them. They are already dead when you try to thaw them out – even though their ‘chemicals’ are all more-or-less in an approximation of their living state.
Wood frogs avoid the problem of ice crystals by the concentration of sugars in their cells. I don’t know how they deal with the suspension of peripheral circulation, though, which is another problem for us – frozen blood carries no oxygen. But there are other issues for us, related to warm-bloodedness. Our biochemistry is ‘tuned’ to 98.4 degrees. But because of the relation of reaction free energy to temperature (ΔG = ΔH – TΔS, T being temperature), then lowering that temperature (or raising it excessively) can catastrophically disrupt a pathway, during the inevitably time-extended process of cooling and thawing the body.
Allan Miller,
This is the whole point. Why should death only be one direction? You are saying the reason the chemicals can’t be alive after we freeze them is because the chemicals became dead. But they didn’t decay. They didn’t really change from what they were. You can claim that the crystals destroyed the structure, and that’s the only reason, but this is just a claim. Claiming that energy is part of the system, and that this is the part that becomes missing is not really a good claim, because we don’t know what gives it this energy to begin with. Food? Oxygen? Ok, just give it that again.
There doesn’t appear to be a single person on Earth who knows what this thing we call life actually is. The materialists argument would be so much stronger if they could just say, you have this bunch of oxygen and carbon and carbohydrates, and proteins, and then if you give it a zap of sunlight and some acids, voila, that is what life is.
But since you can’t we still have to accept the possibility that whatever life is, it may well just be magic to us.
phoodoo,
What hope does AI have for simulating life if intelligence can’t be stopped and started again?
Actually, phoodoo, we understand the role of ice crystals in the fatal nature of freezing quite well. We have done experiments, freezing cells and embryos in the presence of a wide variety of different antifreezes, and then thawing them again. So it is not “just a claim”.
Thanks to the relationship between temperature and ΔG that Allan explained, certain proteins and structures will fall apart as you cool them; so reconstituting them is not simply a matter of warming them back up. You are falling into the Creationist trap he described. The ‘energy’ is present within lots and lots of low* energy bonds; it is not simply a matter of adding it back with a “zap of sunlight”.
*called “high energy bonds” by biochemists who never took chemistry classes.
DNA_Jock,
This sounds suspiciously like the “modern synthesis” BS of evolutionary explanations. Just throw out things and hope some stick. If the claim is that the only hurdle is crystals, I ain’t buying it. One reason we know this isn’t the ultimate answer is because we actually CAN freeze simple cells and unfreeze them and they are still able to live. So if it was all about the freezing then thawing, why does it sometimes work?
So it seems you are just going to try and throw excuses at the problem and hope some sound useful. Just like embryological developmental theory, niche construction, gene flow, gene drift, genetic plasticity …
“Scientists seek to update evolution.” Why oh why would that be necessary, I thought you already knew what evolution was all about??
And I always thought that science never has, and never will, explain anything completely. I take it as a policy position that people will never know “all about” anything, because all things are embedded in a sea of known unknowns and unknown unknowns. I suspect science is a long, long way from understanding everything about evolution, which implies complete knowledge of ecology, genetics, chemistry, reproductive dynamics, and much more.