As discussed here extensively, nothing in “evolution” makes any sense: “natural selection, fitness, speciation, human evolution, gradualism, divergence of character, UCD, TOL, etc. etc.” Not one makes sense. Meanwhile, the “evolution” argument is just one big “affirms the consequent” logical fallacy, while Paley’s excellent argument has never been overturned, and an intuitive intelligent design detector can be used to easily disprove “evolution”. Is there a need for any more proofs? Not really. Are there any other proofs? You bet. Take entropy for instance…
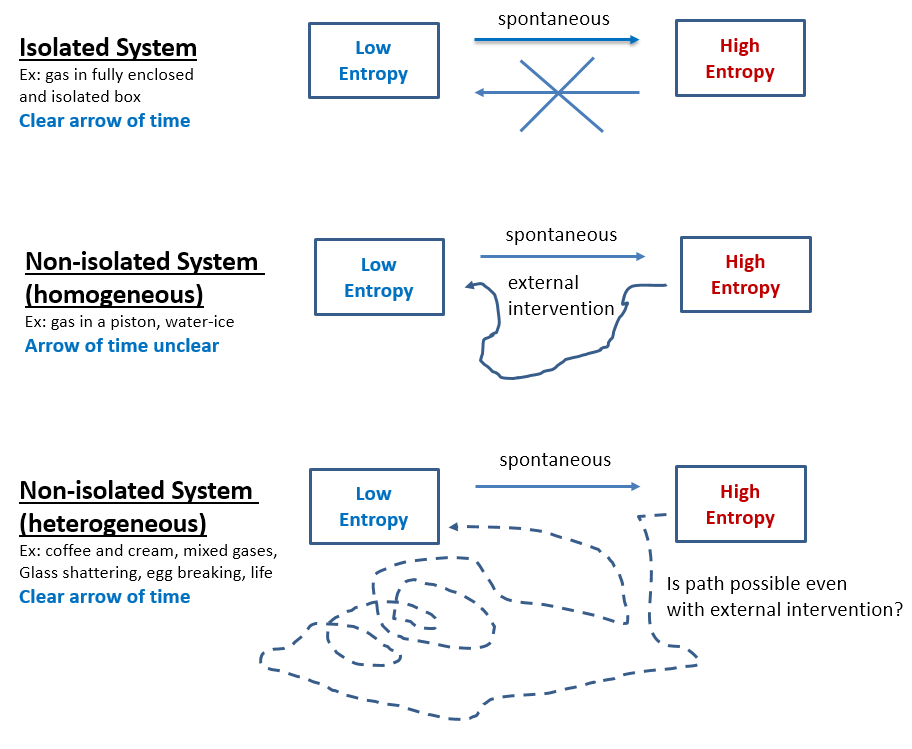
Figure 1
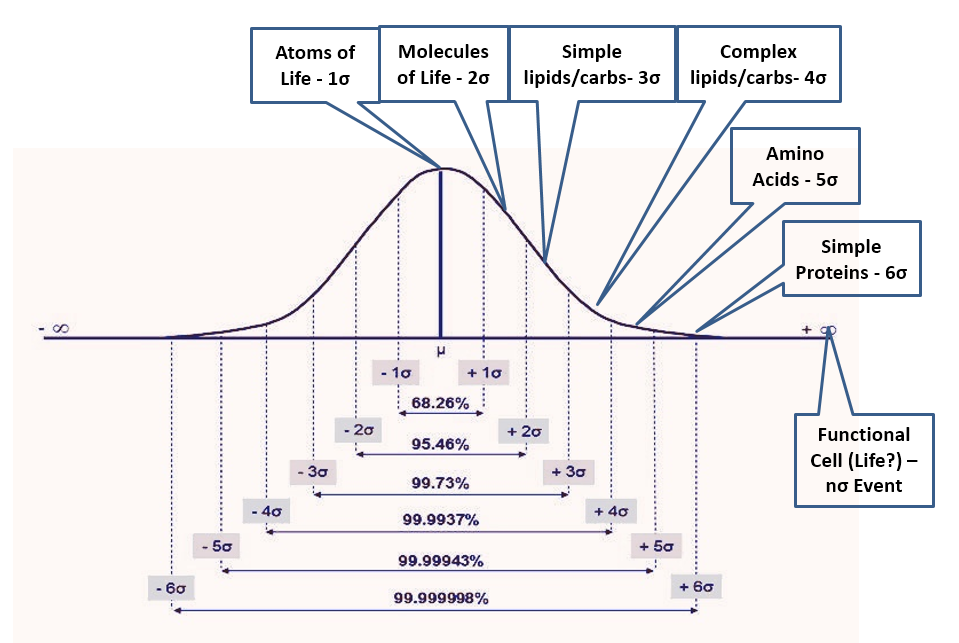
Figure 2
- Second Law of Thermodynamics shows that a spontaneous process cannot also revert spontaneously. This is because spontaneous processes always increase the system’s entropy. A uniform gas in a chamber will accumulate in a corner only with external intervention and spontaneous chemical reactions can only revert if external work energy is applied. Current models of entropy assume the gas particles in a chamber to be independent (sometimes represented as pebbles on a Go board) and explains their never observed convergence on one side of the chamber as only due to that particular microstate having a very low probability(*). However, gas particles always interact with each other (Brownian motion) while pebbles do not. Thus, a reliable way to know that entropy of a system increases is if work energy could be obtained when transitioning from the low to the high entropy state while energy is always required for the reverse process.
- Total entropy of an isolated system can never decrease. Entropy is currently assumed just a statistical law. Thus, if N molecules are in an isolated system (box), the number of microstates associated with j of them being in one half while N-j being in the other half is Ω = N! / (j!*(N-j)!). If N is small, fluctuations seem possible, but before N increases to anything measurable, the probability of fluctuations rapidly decreases to nil. Furthermore, even these theoretical fluctuations, as improbable as they are, might be impossible since the statistical view does not account for molecular interaction observed as Brownian motion and as gas resistance to compression and expansion. Better fundamentals or statistics, either way entropy will never decrease spontaneously in an observable system (Fig 1.a).
- Decreasing entropy is not the reverse process of entropy increasing. That is why a broken egg coming together is easily identified as unreal and a reversed movie of its real shattering. The known laws of physics are the same forward and backward (time-reversal invariance), therefore the reverse shattering process of an egg would not violate any law, but only because these laws are always idealized. Supposedly, if just the right forces are applied to the broken pieces, the egg will come together. In reality this is impossible, and not because the unbroken egg is a highly unlikely microstate, but because entropy increase is not directly reversible even in non-isolated systems. This irreversibility holds for all heterogeneous systems, including life which is perhaps the most heterogeneous system of all. Entropy increase is directly reversible only for homogeneous systems and only if in a defined space. For instance, an expanding gas in an ideal piston creates a force that, when reversed, compresses the gas back into its original state. However, a solid cube of ice can be easily melted by increasing the temperature, but the original ice cube will not reconstitute by lowering the temperature, hence this process too is irreversible despite the cube of ice being homogeneous (Fig 1.b). As far as heterogeneous systems, even separating two mixed gases is way different than the original mixing process, hence mixing is irreversible (Fig 1.c). Entropy decrease is not only different, but also much more complex than entropy increase which is usually spontaneous. Abiogenesis is the entropy-lowering reverse of the biologic decay process, and therefore – if at all feasible – much more complex than adding chemicals and energies.
- Once in equilibrium, a “primordial soup” does not change spontaneously. Life is metastable – it requires certain forms of energy to sustain and spontaneously decays when it no longer receives that energy as well as after the end of the normal lifespan of the organism. It was hypothesized that random fluctuations can spontaneously create compounds and structures given enough time. Abiogenesis, as a reverse-decay process, cannot simply be an outcome of Brownian motion of the chemicals mix because a perpetual motion machine powered by decay and abiogenesis cycles would violate the ‘conservation of energy’ principle. Experimentally, one can confirm that chemical blends in static equilibrium never transition spontaneously into a different equilibrium state (this includes oscillating reactions after the settlement period).
- A “primordial soup” cannot generate life even if energy is applied. It was hypothesized that abiogenesis can be a product of tidal pools, deep sea hydrothermal vents, and the undersurface of ice caps where persistent and abundant energy is available in the form of thermal and electrochemical gradients. Indeed, energy can throw systems off balance and create all kind of chemical compounds and physical structures. However, as the energy applied increases, a complexity limit and hence a dynamic equilibrium is reached where molecule destruction offsets their creation and, if even more energy is applied, molecule destruction dominates, eventually leaving the experimenter with gunk and none of the desired molecules. Miller–Urey and subsequent experiments were not ended because they reached their goal – life – nor because they ran out of energy and materials, but because they reached this dynamic equilibrium, and by adding more of anything would have left them with fewer of the targeted compounds. The amino acids obtained were not the end product but the intermediate between the original molecules and the useless gunk that was the product of the Maillard reaction caused by the energy applied to the system. More complex molecules (and maybe life itself one day) can be created by intelligent designers adding targeted compounds and energies. Then “why can’t natural processes somewhere somehow just mimic the intelligent designer in this vast and almost timeless universe?” The better question is: “why insist on natural processes when the model to be mimicked is that of the intelligent designer?”
- If natural processes were capable of generating life, the environment would be full of intermediate bio-compounds. Life is so complex that laboratories have no hope of replicating it in the foreseeable future. However, if abiogenesis were an outcome of natural processes, the cell structure would be produced only from subsystems and complex biomolecules that in turn would depend on simpler molecules down to H-C-O-N, the atoms of life. A “primordial soup” capable of generating life, thus must contain all intermediate compounds from the atoms of life to the most complex biomolecules and subsystems in an ever-decreasing ratio as complexity increases. Not knowing anything about how this process would work (or even if possible), the most reasonable assumption is a normal distribution of outcomes with life being an n-sigma event (with n unknown) while the availability of the atoms of life being a 1-sigma event and anything else falling in between (Fig 2). Many x-sigma events would be required for each (x+1)-sigma event, with a good first approximation given by the normal density function. Thus, the 2-sigma event could be the basic molecules of life (water, methane, etc.), and we would expect only one of these events for every seven of the 1-sigma events. This approximation would further yield (in one scenario) 1/7 fewer molecules of life than atoms of life, 1/17 fewer simple lipids and carbohydrates molecules (3-sigma) than of molecules of life, 1/43 fewer complex lipids and carbohydrates (4-sigma) than 3-sigma events, 1/110 fewer amino acids (5-sigma) than 4-sigma, 1/291 fewer simple proteins (6-sigma) than 5-sigma, 1/771 fewer complex proteins (7-sigma) than 6-sigma and then – rule of thumb – 1/1600 (8-sigma), 1/3800 (9), 1/9100 (10), 1/22k (11), 1/52k (12), 1/126k (13), etc. fewer of each additional sigma event than previous event where 8+sigma being (this scenario) nucleic acids, short chains, long chains, organelle subsystems, organelles, other critical cell components and finally the fully functional biologic cell – the n-sigma event which is not quite life but good enough for this analysis. Then how can we test this?
- Apart from life itself, the complex molecules of life are nowhere to be found in the universe. To test the ‘natural processes’ hypothesis of abiogenesis, one must observe the intermediate components of life in nature and in the ratios estimated above (or from another reasonable estimate). In addition, one must observe the spontaneous transitions (aided by energy) from simple to complex even if not all transitions are observed at once. Earth is “polluted” with life down to the deepest ocean trenches, therefore the first focus is the extraterrestrial space where, too bad, the largest confirmed interstellar molecules have a maximum of 13 atoms (apart from C60/C70 fullerene). Back on earth we see all intermediate components, but only within life itself. Outside of the cells, aside from the simplest biomolecules, we only see products of decomposition that are never in the ratios associated with abiogenesis, meaning we never see increasing molecule complexity in decreasing ratios resembling anything reasonably expected. Abiogenesis is not happening due to the irreversibility of the entropy increase and for the same reason egg breaking, butter melting, gas mixing, etc. are not reversible processes. Humans can only create a few of the complex molecules, although most always aided by life itself, and even then the power of synthetic biology is severely restricted. The more complex, the harder these molecules are to obtain and the faster they decay instead of spontaneously combining with one another to form even more complex compounds and ultimately life.
- Miller–Urey style abiogenesis experiments are ill conceived, hence doomed from the beginning. To be more specific, they are only good for PR (public relations) given the irrelevant “organic compounds” created that raise the hopes of the believers. Trying to obtain an automobile from scratch by mixing chemicals and energy, qualifies the person attempting as delusional and the one selling such vision as charlatan. So why would those attempting the same with life – which is infinitely more complex than an automobile – not also be labeled charlatans and delusional? Abiogenesis experiments belong to the Reverse Engineering category of processes and, when done right, they are very different than Miller–Urey. Their starting point is never some “primordial soup”, but the most advanced compounds available, preferably already organized in working subsystems. Swapping organelles or parts within organelles, exposing organisms to various environments, attempting to revive dead organisms, substituting engineered subsystems and so on are part of the hard work with long tradition and already being done in medicine and many industries for other purposes than to prove abiogenesis. If and when someone will be able to reverse the decaying and dying processes, we will know that abiogenesis is possible as an act of Intelligent Design creation. To confirm abiogenesis as an “unguided process” we would have to observe reverse-decay and reverse-dying processes happening in nature, not in a lab. Yet 2nd law proves this impossible.
- Is abiogenesis not feasible because it was a unique event? If true, abiogenesis would be a “materialistic miracle” and furthermore not just one, but a long series of “materialistic miracles” since a long series of – so far unknown – events are needed to get from atoms to the simplest organism. Yet one of the tenets of materialism is “no miracles” showing the inconsistency of the materialistic “unique event” assertion. And of course, physics and chemistry transformations are never unique. And even if entropy allowed for abiogenesis, the laws of life do not follow from any priors (physics, chemistry, mathematics). Life has a drive to survive and leave off-springs which entails harm avoidance, immune system, metabolism, food seeking, homeostasis, growth, reproduction, and body structure. Without these, any cell would start decaying the instant it was formed as in fact it does as soon as it no longer is alive. Despite having lasted almost since the formation of The Earth, life is metastable – one knock and it dies and then decays. This is unlike other negative entropy machines that can be restored (rebuilding proportional with the damage).
- Other considerations.
- “Dissipation-driven adaptation of matter” (J. England, MIT) claims that life is inevitable because life “absorbs and dissipates more energy from external sources” leading to faster entropy increase. However, there is no law that entropy has to increase faster. In addition, most of the entropy in the universe is captured by black holes with life having a nil contribution to that entropy.
- Some claim they have obtained “protocells” that seem to mimic real cells at least in part. However, “protocells” are to biological cells as fool’s gold is to real gold.
- “Kolmogorov complexity is lowest at low and high entropy and high in the middle hence life is supposedly inevitable (S. Carroll)”. However, life is not complexity. Life is much more than snowflakes, vortices and chemical reactions (candle burning). And most certainly, life is not the complex swirls of cream mixing into coffee on a journey from low entropy to high entropy (both having low complexity). In addition, unless very specific external action continues to be applied to maintain those patterns, they soon disappear like in sand dunes exposed to shifting winds. The patterns therefore do no “arise”, but are created by an external force.
- “Gradients of energy in deep vents are responsible for abiogenesis”. But all organisms from these exotic places are very similar to any other ones found elsewhere, hence all likely have the same origin. In addition, no free floating organic compounds (aside from decay byproducts) have been found there to suggest ongoing abiogenesis. And, aside from the simplest molecules, no spontaneous transitions from x-sigma to (x+1)-sigma bio complexity has ever been observed around these deep vents either.
- Of course life does not violate 2nd Organisms do conform to 2nd law when they decay as soon as they die. In addition, as observed by Erwin Schrödinger, “the increase in entropy from turning our low-entropy food into our high-entropy waste is greater than the local decrease in entropy from making the well-ordered structures within our bodies”. Nothing special so far – a refrigerator does the same: creates a zone of low-entropy while the entropy of the whole system increases and for as long as it’s fed energy.
- Randomness can theoretically account for any bizarre occurrences including Paley’s watch and F. Hoyle’s 747 in baby steps if enough time is given. But no such event was ever observed. In addition, breaking down the unattainable complex system into a combination of simpler components, each with higher probability of occurrence makes it no easier as the probabilities of all subsystem have to be multiplied to get back to the complex final assembly.
- Some claim that life itself prevents abiogenesis by ingesting all intermediate molecules spontaneously formed, but this can be easily prevented in sterile labs. In addition, all complex intermediate molecules observed outside of cells are due to decomposition, not abiogenesis.
- “Evolution” corollary number 1. If abiogenesis is impossible as an undirected, natural process, then whoever is responsible for abiogenesis is also responsible for the biologic landscape past and present, therefore “evolution” is also impossible as an undirected, natural process.
- “Evolution” corollary number 2. It is easy to verify that nothing ever “evolves” in the nonliving nature. Life is said to be “just chemistry”. These two combine to: nothing “evolves” in the living either. Solar systems, geographical features, fluid eddies, chemistry, snow flakes, etc. all go through their life cycles, and all are different from each other, but the life cycles of the newer entities are no more “evolved” than the life cycles of the ancient ones.
- “Evolution” corollary number 3. Presumably, “evolution” has not ended. And if ongoing, then one must see the normal distribution of the different transitioning organisms (the intermediary), just as we would see if abiogenesis were true. If humans evolved from monkeys and “evolution” is ongoing, then humans must still be in transition especially since the human population is one of the largest of all mammals and, the more individuals, the more “evolving” opportunities. The older Darwinists replied with a hierarchy of races. But that reply is not only fashionably repugnant, but also false and, amazingly, contrary to [at least] the Abrahamic religions that have always known better.
- In conclusion, abiogenesis is nothing more than the decay process running backwards, therefore easily visualized, yet impossible according to the second law of thermodynamics. In other words, “evolution” is nothing more than imagination run wild. Expecting abiogenesis to be within reach if only the proper forces and chemical compounds were added is as wrong as expecting the broken egg to come back together if only the proper sequence of forces were applied to the broken pieces.
Summary:
- A spontaneous process cannot revert spontaneously.
- Mixtures will never ever spontaneously separate per second law.
- Decreasing entropy is not the reverse process of entropy increasing and also much more complex.
- Once in equilibrium, a “primordial soup” does not change spontaneously.
- A “primordial soup” cannot generate life even if energy is applied due to dynamic equilibrium.
- If natural processes were capable of generating life, the environment would be full of intermediate bio-compounds.
- Apart from life itself, the complex molecules of life are nowhere to be found in the universe.
- Abiogenesis experiments belong to the Reverse Engineering category of processes.
- Miller–Urey style abiogenesis experiments are ill conceived, hence doomed from the beginning.
- Abiogenesis unique event conflicts with the “no miracles” clause of materialism.
- Even if entropy allowed abiogenesis, the laws of life do not follow from any priors (physics, chemistry, mathematics).
- “Evolution” corollary number 1 – no abiogenesis, no “evolution”.
- “Evolution” corollary number 2 – no “evolution” in the inert and “life just chemistry”, then no “evolution” in the living.
- “Evolution” corollary number 3 – no intermediate “evolving” entities, no “evolution”.
- Being a decay process running backwards, abiogenesis is as impossible as a broken egg being reconstituted by the “proper sequence of forces”. “Evolution” is also nothing more than imagination run wild.
(*)R. Penrose “The Emperor’s new mind”; PBS SpaceTime “The Misunderstood Nature of Entropy”; Sean Carroll “From Eternity to Here”, etc.
Links:
Abiogenesis: The Faith and the Facts
James Tour: The Mystery of the Origin of Life
Chirality, Maillard – caramelization, characterize the structure at every step:
https://creation.com/why-the-miller-urey-research-argues-against-abiogenesis
https://evolutionnews.org/2014/06/squeezing_the_l/
https://www.ncbi.nlm.nih.gov/pubmed/21422282
Entropy of a box of molecules
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2253472/ https://en.wikipedia.org/wiki/Normal_distribution#Cumulative_distribution_function
http://physics.bu.edu/~redner/211-sp06/class-engines/class25_secondlaw.html
https://www.quora.com/How-quickly-is-the-entropy-of-the-sun-changing
https://www.thoughtco.com/how-many-atoms-in-human-cell-603882
https://www.amazon.com/Mysteries-Modern-Physics-Sean-Carroll/dp/1598038699
https://en.wikipedia.org/wiki/File:Elements_abundance-bars.svg – abundance in the solar system
https://en.wikipedia.org/wiki/Miller%E2%80%93Urey_experiment
https://en.wikipedia.org/wiki/Proteinogenic_amino_acid
https://en.wikipedia.org/wiki/Artificial_gene_synthesis
https://www.scientificamerican.com/article/a-new-physics-theory-of-life/
https://en.wikipedia.org/wiki/Dissipative_system
https://pubs.usgs.gov/gip/dynamic/exploring.html

So the number of faces on the die is irrelevant, and the number of dots on each face of the die is irrelevant, and the number if die is irrelevant. So let’s forget about configuration entropy.
What is the temperature. That is all that matters.
So if we simply measure the temperature of each die we can determine the distribution of energy quanta. You don’t believe that, and neither do I.
Why not admit that you are peddling nonsense?
Ah. Distribution of Energy Quanta Entropy. You have a mathematical formula that incoporates this into statistical mechanics?
To me, “Distribution of Energy Quanta Entropy” sounds like a made up name. You were never skeptical?
I’ve never even heard of “the Flanders and Swann song.” So can’t say whether I understand it. If you have previously posted about it here at TSZ and emphasized it’s relevance I’d like to read your comments. Please post a link.
I admit I don’t know what that phrase refers to, since this is well outside my knowledge base. But I nonetheless presume it means something. Skepticism doesn’t mean assuming others are as ignorant as I am; that if I don’t know what it means, it must not mean anything.
You don’t know what it means. You assume it means something. But you don’t know what.
Hey, Mung. See this comment for the link you requested.
Read the preceding page (page 5) of comments to understand the context. You might want to review Prof Lienhard’s video, too, as that’s what I was reacting to. Hope this helps.
I want you to defend your claims, in your own words. Isn’t that what you demand of others? Demonstrate your competence.
You base your beliefs on something you watched on YouTube?
If you want me to “defend my claims, in my own words”, Mung, you will need to be somewhat more specific. Page 5 of this thread has nearly a thousand words I wrote, explaining to nonlin why he is wrong about “entropy forbids abiogenesis” in my own words.
It appears to me that you are disputing my claim that heat and temperature matter. I have been explaining to nonlin how entropy changes due to heat flow are, in general, large when compared with the sort of ‘configurational’ entropy change beloved of creationists.
In your question about two dice, I asked
“How warm are these dice?”
You replied
“Why does that matter?”
I explained that the number of microstates is gonna depend on the temperature.
Your response
Classic Mung. Sad, frankly: I never said the number of faces on the die is irrelevant; I said the temperature matters.
But we can ball park how much. Suppose a single photon is absorbed by one of the dice. That energy can be transferred to excite any of the atoms in that die. So for a typical die, maybe 10^22 different ways.
The physical configuration of your six-sided die matters. Just not a lot. I thought that this fact was well illustrated by Prof Lienhard’s demo that only involved 6 quanta and 8 atoms: the low entropy starting state corresponded to 4 x 56 = 224 microstates.
Now, if you actually want to have a conversation about thermodynamics, you will also need to state WHY you dispute specific claims; the Socratic method only gets to conversation so far — at some point you will have to state what you think, and why. You generally avoid this like the plague.
You carefully omitted the part where I said that, I don’t take it for granted that if I don’t know what it means, it must not mean anything.
You seem to be saying “I don’t know what this phrase refers to, therefore it must not mean anything.” This is known as the idiot’s conceit.
And I have. Take the hot magma/melting ice most recent nonsense you produced. Your silence speaks for itself. Meanwhile, you’re only disliking what I say but never follow through with evidence.
I see you fold on my charge your understanding is primitive – finally a smart move.
Since you insist, and since you’re speechless, here’s what’s wrong with the video: (min 10:30) he splits the number of microstates equally between the two systems. That’s correct given thermal equilibrium even if his number (20) is not right. But for the same exact reason, his initial hot system (min 10:00) does not have 56 microstates. That is because this system is also in equilibrium which means only evenly split states like (1 1 1 2) and (2 1 1 1) are valid. It could also be in transition (not his thinking) which means only unbalanced states like (5 0 0 0) and (4 1 0 0) etc. are allowed. If we consider a hot iron rod, (1 2 1 1) is even heat, while (0 0 0 5) is uneven (hot spots) and these two microstates represent different macrostates.
Now, here’s your chance to shine… Agree? Disagree? Understand? Clueless?
Which probably means kinetic energy (heat). But there are more energy forms.
He probably means hot air which is the only thing he produces.
Hence my biofuel challenge to your melting ice engine. Do you understand the value of chemical energy? Why are we using it preferentially? Why do you power your life with biofuel in the form of fossil fuel instead of the hot air you produce?!?
And back to abiogenesis, we’re not just interested in energy density: https://transportgeography.org/?page_id=5837
Many other facts prove abiogenesis impossible:
1. A spontaneous process cannot revert spontaneously.
2. Mixtures will never ever spontaneously separate per second law.
3. Decreasing entropy is not the reverse process of entropy increasing and also much more complex.
4. Once in equilibrium, a “primordial soup” does not change spontaneously.
5. A “primordial soup” cannot generate life even if energy is applied due to dynamic equilibrium.
6. If natural processes were capable of generating life, the environment would be full of intermediate bio-compounds.
7. Apart from life itself, the complex molecules of life are nowhere to be found in the universe.
8. Abiogenesis experiments belong to the Reverse Engineering category of processes.
9. Miller–Urey style abiogenesis experiments are ill conceived, hence doomed from the beginning.
10. Abiogenesis unique event conflicts with the “no miracles” clause of materialism.
11. Even if entropy allowed abiogenesis, the laws of life do not follow from any priors (physics, chemistry, mathematics).
12. “Evolution” corollary number 1 – no abiogenesis, no “evolution”.
13. “Evolution” corollary number 2 – no “evolution” in the inert and “life just chemistry”, then no “evolution” in the living.
14. “Evolution” corollary number 3 – no intermediate “evolving” entities, no “evolution”.
15. Being a decay process running backwards, abiogenesis is as impossible as a broken egg being reconstituted by the “proper sequence of forces”. “Evolution” is also nothing more than imagination run wild.
Oh dear.
Working, as I was, from the transcript, I did not realize that Prof Lienhard actually provided the answer too. I was hoping to test nonlin to see if he could calculate the number of macrostates corresponding to 3 quanta and 4 atoms. Prof Lienhard told him that there’s 4 ways to distribute 1 quanta and 56 ways to distribute 5 quanta (4 @ 5000, 4 @ 2111 and 12 each @ 2210, 3110, 3200 & 4100)
That’s why I asked nonlin:
Now, I did think there was a reasonable chance that nonlin would be able to figure out that there are 20 ways to distribute 3 quanta to 4 atoms (4 ways to do 1110, 4 ways to do 3000, and 12 ways to do 2100)
But I was quite confident that nonlin would be unable to understand the concept here: that he needs to multiply the number of microstates possible in each bar to come up with the number of microstates for the system. I gave him a truly massive hint when I wrote
And the take home message is that 20 x 20 = 400, which is larger than 4 x 56 = 224. And in any practical setting you will find that the number of microstates (consistent with the macrostate) hits a maximum when the energy is distributed evenly. And THAT is why you “can’t pass heat from the cooler to the hotter” [What the MIT Prof does in the video is to take log W, then add. Hopefully nonlin is aware that log(AxB) = log(A) + log(B).]
Unfortunately, even when fed this correct answer, nonlin cannot understand it.
Truly bizarrely, nonlin writes
NO HE DOES NOT SPLIT THE NUMBER OF MICROSTATES EQUALLY BETWEEN THE TWO SYSTEMS. That makes no sense at all.
He splits the energy equally. And of course Prof Lienhard’s answer of 20 is correct. Does nonlin think it should be 30?
Ack!
[Note for grown-ups: in the sense that W stands for Wahrscheinlichkeit, and not the number of macrostates, we should really be calculating rho.log(rho), so there is a level of sophistication at which 5000 may not always be quite the same as 2111. But nonlin ain’t there yet.]
Even when fed the correct answer for the number of ways to distribute 3 identical objects into 4 buckets, nonlin still fails at High School sophomore math.
Nonlin.org,
All of that was already taken care of ad nauseam. Even you decided that you were all wrong. So you must be authentically desperate.
Of course, you stoped answering me because I make it too obvious that you have no idea. You twist yourself into extraordinary contradictions, then you twist yourself even more, then you try to untwist yourself, only to twist even more. You’re entangled without any way out. It’s fantastic. Yet, you claim you’re “100%” right, as if you didn’t know that contradictory claims cannot both be true (you didn’t know?! oh, sorry, I knew you’re illiterate and nonsensical, but didn’t know it was that bad). Not only that, you’re contradicting yourself with that very claim, since you don’t even believe that there’s such a thing as 100%!
Amazing.
Nonlin.org,
You’re just regurgitating your already-addressed tripe here. It’s not ‘decay running backwards’, not restricted to ‘soup’, does not require any exceptional reversal of the entropic ‘arrow’, etc etc. This is all just one massive flail.
As discussed.
Sure I’m in denial. I don’t accept your argument. To the extent I am going in circles, it is because you are – kind of like a perpetual motion machine.
You haven’t ‘proven’ that abiogenesis is decay running backwards, so there is certainly no onus on me to prove it isn’t. You appear, from a position of almost total ignorance, to think that if ‘decay’ is a process with negative ΔG, anything one might declare to be its ‘opposite’ must have a positive ΔG. That would only be true if they followed exactly the same reaction path.
Prove it! Prokaryotes achieve osmotic homeostasis passively. So evidently active homeostasis, requiring the input of energy, isn’t ‘primordial’.
The weakest possible response is what you went for? Fantastic work. Sure I’m in denial. Because you’re wrong. Gosh, this is easy!
Another phrase picked up off the internet and misapplied. Wrong. Metabolism of food and decay of faecal matter are both ‘forward’ processes, entropically and temporally. From a bacterial standpoint, decay of faecal matter, and bodies, are both ‘eating’. It would only be entropic reversal if the bacteria somehow regurgitated a body. Which is how you seem to see abiogenesis. Yes, I can agree that seems rather … uh … improbable. But since that is an incorrect viewpoint, it need not trouble us.
Not seen that from anyone but you.
What, some dead guy? 🤣 No, but there is more to entropy than statistical mechanics.
Which it is, provided one remembers that ‘local’ entropy decrease is invariably coupled with a more widespread entropy increase. It happens every time you eat a sandwich. It is trivial, and requires no violation of 2loT.
They are both expected to be entropically and temporally ‘forward’, yes.
No, they think A-to-B is not the reverse of B-to-C, in chemical terms. Only by casting things in the vaguest possible terms do you get a ‘C’ that is the same as an ‘A’. By ignoring chemistry, IOW, and making such howlers as considering all equilibria as equivalent.
You can only decrease the entropy (locally) by dispersing some of its energy, statistical reversals aside. Doesn’t matter if it’s a hot gas, a folding protein, a reaction with negative ΔG, a bacterium chowing down on your discarded skin cells or you cramming another burger in your gob.
(‘Study’? HAHAHAAAA!)*** Yeah, classical entropy is SO last-century (or the century before that).
*** eta – I wrote that before reading Entropy’s almost identical comment! I cede priority.
Taking the questions at face value … It matters more generally in relation to the teaching of thermodynamic concepts. Lambert was concerned that teaching it as ‘order change’ leads directly to the kind of tosh we are seeing here; an emphasis on configuration and ignorance of energy. More directly in relation to you, I ask because you were apparently making some point about Lambert, by reference to ‘his critics’.
Yet in response to a statement about Lambert ‘making sense’, you make reference to his critics. That implied some grasp of the area, the pros and cons of Lambert’s view.
A polypeptide immediately prior to folding. A tyre at the moment of puncturing. A hot metal bar in a cooler room.
@ nonlin. This assessment is accurate. Consider that nobody who has read your stuff here or elsewhere has taken it seriously. Repetition of your failed arguments is not going to change anything. So I ask again:
The next step?
As far as I can see, for you there is no next step.
Alan Fox,
I think that in Nonlin’s mind, if she just reposts the same crap, and we don’t bother to repeat the dismantling, then the already failed arguments “stand undefeated.” Maybe Nonlin imagines that the prior slaughter of her nonsense never happened, because they were written before the reposting of the same failed crap.
Nonlin has a very weird, twisted, mind. I would not be too surprised if this was her “reasoning.”
Yes and non!lin is far from unique in claiming “victories”. But where is nonlin’s support? There is no fan club. (S)he is a maverick who is too problematic even for anti-evolution groups. I agree the repetition is irritating and I wonder what the attraction is for nonlin. Not being ignored, perhaps?
Haha. I’m still counting the number of times you think you got something and end up having nothing. When will you learn? But keep trying.
In this case, split energy is split states, so either is correct. And the rest of what I wrote, flew right over your head. Whoosh!
Here’s what you missed:
Initially, not all microstates he counts represent the same macrostate. So for system 1, there’s only four combinations of the type (2 1 1 1) whereas system 2 is right. IOW, for system 1, a combination of the type (5 0 0 0) that he counts as valid, is in fact NOT valid. Yes, I know the formula, but the formula is wrong. So combined number of microstates is 4 x 4, not 4 x 56! Once the two systems are in contact, the energy is assumed to flow freely, so no, there aren’t two systems, but a combined system. And for that, we have C(8,6), meaning 28 microstates for the combined system. Because again, any other microstate is NOT compatible with the macrostate. So a (4 0 0 0 2 0 0 0) is not valid.
You mean microstates. No one is counting the number of macrostates. Anyway, not only is 5000 not the same as 2111, but they do not represent the same macrostate. How so you ask? I already explained that in the context of a hot iron rod, (1 2 1 1) is even heat, while (0 0 0 5) is uneven (hot spots). These two are different macrostates and the second one doesn’t even represent equilibrium. Whoosh again!
🙂
Allan Miller,
Yet there is no abiogenesis anywhere. WHY?
Abiogenesis suffers from the same exact problem as “evolution”. As per Fig 2, there is no evidence of intermediate steps happening. Ever. Just as there’s no “gradualism” anywhere to be observed in “evolution”, there are no abiogenesis intermediate compounds anywhere. With all the money spent on research, humans should AT A MINIMUM be able to re-create some of the steps in abiogenesis. We should not rely on bacteria to make insulin and we should be able to design artificial organs instead on relying on transplants. Even if human design is NOT at all abiogenesis. But as they say “directed evolution”… what a sad and stupid joke!
But you are “certain”. How can that be if you’re asking me to do something?!? Whatsamatter? Logic 101 is too hard for you?
Active, passive, who cares? It is homeostasis for sure. And it’s primordial. Problems with Logic 101 again? Ouch!
Lighten up and take a joke! You’re still full of it. Haha.
Sure it happens. And sure it doesn’t violate 2nd law. But is it TRIVIAL? No, it isn’t! Damn Logic 101 again?
But they’re fundamentally different! Agree? Never mind. They ARE different if you agree or not. Everyone knows.
Then let’s explore this. If B is a bio cell and A is its composing molecules and C are the basic molecules resulting from the decay of B, how is C DIFFERENT than A? Quick! I’m holding my breath!
Then where is chemical energy coming from if all is dispersed? I’m cool with “some of it”, but it’s not clear what he meant: http://theskepticalzone.com/wp/entropy-forbids-abiogenesis-evolution/comment-page-5/#comment-282079
But this topic isn’t “entropy”, is is? NO! It’s “entropy forbids abiogenesis”.
Back to “vote for science”? You just don’t get it, do you? Of course you don’t, it’s just rhetorical…
You’ve got it wrong at a few levels, so let’s see if an illustration helps you out: starch can be “broken” into smaller chains, up to glucose, by hydrolysis, a process that “uses” a molecule of water that’s incorporated into the liberated compounds. The “backwards” process proper is called “condensation” because it would free molecules of water. Since that cannot easily happen in aqueous solution, biosynthesis of starch doesn’t use condensation, but, rather, it uses “charged” glucose, glucose with an ADP bound to it. This ADP-glucose compound has enough energy that it can be exchanged with a glucose-glucose bond in an energetically favourable reaction. Of course, that ADP-glucose results from yet another set of energetically-favourable reactions, and so on.
So, starch is not formed following the same reactions of its “decay” in reverse, but by a different pathway of reactions, all energetically favourable, all in accord with the second law of thermodynamics.
Let’s see you dance.
So nonlin cites a video by a Mechanical Engineering prof, describing it thus:
but nonlin maintains that said MIT prof is actually incapable of counting microstates correctly.
Wow.
Interestingly, according to nonlin-physics the entropy for a 4 atom system looks as follows:
# quanta ==> entropy (kb)
0 ==> log (1) [this, at least , is correct]
1 ==> log (4) [so far, so good]
2 ==> log (12) [a little off]
3 ==> log (4) [huh, the entropy went down as we added heat. The temperature is negative now?]
4 ==> log (1) [WTF, there’s zero entropy now??]
5 ==> log (4) [this one he quoted explicitly]
5 ==> log (12) [rinse lather repeat…]
This is absolutely hilarious. There’s whole new levels of misunderstanding here. Alan is right, the only thing left for nonlin to do is to publish this seminal overturning of TD.
Entropy,
My answer was going to be “less RNA”.
🙂
Local entropy decrease is going on all around you. You couldn’t fail to see it happening. Just trying not to see it happening would be a demonstration of it happening. I’d call something like that trivial. If it wasn’t trivial it would be very strange to find anything like that going on. You and your silly, stubborn, arrogant ineptitude wouldn’t be anywhere to be found.
That’s what makes your position so absurd. So much local entropy decrease is going on all around you. It’s even going on inside you, making your own body and your very nonsensical thoughts happen, and you’re still going on tantrums after tantrums that we call them trivial. You couldn’t be any more clueless if you were doing it on purpose. Damn logic 101 indeed.
Yes, I’m certain. Decay is performed by bacteria, fungi and invertebrates. ‘Decay running backwards’ would be those reactions running backwards – bacteria regurgitating a zebra. That doesn’t happen, and no-one argues it does.
No, it isn’t. See? Just Saying Stuff is so easy.
Just because you didn’t see me rolling round the floor clutching my sides at your hilarity, doesn’t mean I wasn’t. Tee hee. Nonetheless, you also claim to speak the ‘language of science’.
It is entropically trivial. That’s all it needs to be in relation to your specific thesis.
Yes, they are ‘different’. It does not follow that, being ‘different’, one is entropically forbidden and the other isn’t.
If the final state C were the precise same configuration as in A, then yes, C and A would be ‘the same’. However (in a parallel of entropic microstates) there are vastly more states C that are not ‘the same’ as A, even given the same basic atoms (which is itself not a given). Therefore picking a state C and saying “that must also be state A” would be unjustified. It would be even less justified to declare that the path B-C was necessarily the same as the path A-B precisely reversed.
For comparison, the sandwiches, pies and beer (state A) that now constitute my body (state B) differ from the atomic configuration that will ensue following my demise (state C), and follow different reaction paths. Only a clown would insist that B-C was the same as A-B. However, some clowns have decided that, in the initial formation of a B. It was the precise reversal of B-C. I don’t see why that follows from any principle.
It ‘disperses’ as part of the reaction – as part of the process of entropy change.
If you can’t/won’t understand the basics of entropy, anything that follows is just you talking shite.
Nonlin.org,
I’ve told you why I think that is: Tenure. The conditions in a biologically rich world are different from those in a sterile one. Prokaryotes in particular are very good at incorporating food sources. Once life gets established, conditions are less favourable for it to happen again, or at least get to a threshold of detection, by that fact alone.
You don’t buy it, and you don’t have to. You’d have someone create new life and see if it’s competitive, which is a pretty dumb rebuttal. Despite that, it remains a perfectly rational answer to the question.
We already agreed to compare only beginning and end results to determine what’s the reverse of what. Intermediate steps can be different and A to B is still the reverse of B to A.
You still haven’t learned that adding energy can reduce local entropy?!? And of course, zero entropy is perfectly valid.
You must be one of them slow learner – fast forgetter. Didn’t I teach you that biologic entropy is very low? Remember the hot magma/frozen ice fiasco? What about the need to normalize? Do you get it now, or did you forget that lesson already?
Not much in the inert. Meaning NOTHING compared to life. Abiogenesis is BEFORE life, so look only at the inert.
Yes, but proving is difficult. I show you homeostasis in 100% of life. You only need to show ONE counterexample. Go ahead.
My thesis is “entropy increase is trivial whereas entropy decrease is NOT”. You’re retorting with “It [entropy decrease] is entropically trivial” which is a retard claim.
Of course it doesn’t follow from the difference [life vs abiogenesis]. But the retard claim was “if life is possible, then abiogenesis is”.
“Sandwiches, pies and beer” are not the end points (basic molecules), but intermediate compounds.
“Six elements [CHNOPS] account for 99% of the mass of the human body.”
If you decay a human, you end up with these (net!). To build a human, you need these in the same quantity. Within tolerance. No more, no less. QED.
Look, for the local entropy to decrease, it is imperative that LESS than 100% of the energy to be dispersed in the environment.
1. Yet they are trying abiogenesis in the lab. Clearly I’m not the only one not buying your “tenure” nonsense.
2. Humans can and DO produce sterile lab environments. Miller-Urey was not ended because prokaryotes ate their lunch. Instead, their output turned into gunk. Because entropy increase is the norm.
3. There should be SOME evidence of incipient abiogenesis, the products of which would be ingested by prokaryotes.
4. Even if prokaryotes ingested 99.9(?)% of the products, there should still be traces of said products of abiogenesis
5. We need not test the steps in order: 1,2,3,4,… We can (and DO) also test 6 to 7 or 4 to 5 or anything we want. They all fail. Every single one.
(I thought you would dance, and lo and behold, you’re dancing.)
I never agreed to such a thing. However, had we agreed, your “point” would have been immediately invalidated and “reverse-decay” would not be against entropy.
So, either
a. You keep your “nomenclature” to accept that abiogenesis could proceed by pathways of entropically favourable reactions, thus renouncing your claim that “reverse-decay” is forbidden by entropy,
or
b. You renounce your “nomenclature” to accept that abiogenesis could proceed by pathways of entropically favourable reactions, and keep your claim that reverse-decay is forbidden by entropy.
In both cases one of your claims is invalidated, and entropy doesn’t forbid abiogenesis given that the pathways are different, whether you want to talk about them by the same “name” in “reverse” or not. So, take your pick.
In the “inert” it’s also happening all the time. Right now it’s snowing here, and all that snow was formed by local decreases of entropy. Lo and behold! The mixture of water and air separated spontaneously as the temperature dropped, and the water molecules reordered themselves and grew into these nice crystals that are falling all around me right now.
It doesn’t take much looking around to see “non-life” getting those local entropy decreases too. So, yes, I’d call that trivial. If you don’t want to call that trivial, that’s all right by me, as long as you’re aware that playing with definitions doesn’t change the fact that, if there’s a problem for abiogenesis, it certainly is not entropy.
That being said, merging a 3quanta-4particles with a 2quanta-1particle does seem to lower entropy spontaneously. And that is the problem with these thought experiments: they don’t always make sense and generally can’t be verified experimentally (that’s why they’re only “thought”).
Anyway, the bottom line is that not all microstates imagined are valid. And with regards to “entropy forbids abiogenesis”, it is clear that there are no significant spontaneous fluctuations to facilitate abiogenesis. IOW, equilibrium rules, and it rules AGAINST abiogenesis. Like I wrote in par 2: “Better fundamentals or statistics, either way entropy will never decrease spontaneously in an observable system (Fig 1.a).”
Who cares? It is what it is. A-to-B and B-to-A are the reverse of each other. And when it comes to entropy there’s a strong asymmetry. One that can easily be verified and that forbids abiogenesis.
I agreed no such thing. We don’t know what the beginning is, but there is no reason to suppose it is the same as ‘the end’. More than that, if you are talking of reversibility, you are talking of paths. To ignore them , or to call two different paths reversals of each other, would be absurd.
Even you should see that looking at modern life and deducing that its characteristics are definitively ‘primordial’ is shaky logic. After all you claim to have passed Logic 101.
Local entropy decrease is trivial. Look at crystallisation, for example. We are making no appeal to situations where the overall result is a decrease with no wider increase.
That wasn’t the claim. I’m simply responding to ‘they are different’, as if that justifies an entropic distinction. If life works by following thermodynamic gradients – through processes of local entropy decrease accompanying wider entropy decrease – then that same relationship is likely to be – uh – primordial.If the end result of abiogenesis is a living organism locally decreasing entropy, I see no a priori reason to suppose that that started only at the moment abiogenesis ended. Even the simplest of chemical reactions can have that character, of local entropy decrease and wider increase, so there is no reason to make abiogenesis an exception to principles operating in both chemistry and biology.
As far as my personal system is concerned, speaking as a B, they are the ‘A’ in your A-B-C malarkey. If A-B is not the reverse of B-C in that system, I don’t see why it should be in one where Bs arise abiogenetically.
Nope. The inputs do not have the same composition as a human. That is absurd. And there is more to us than CHNOPS.
Depends what you think ‘the energy’ is. If an object falls to earth, ALL of its potential energy is dispersed, with local entropy decrease. Of course it still has mass-energy, but I doubt that’s what you were talking about.
I’m not aware of such a researcher who thinks that abiogenesis should be vanishingly rare. That would seem to make their pursuit particularly pointless, if they have zero confidence in it! So they may well buy it, if they think abiogenesis common enough to be replicable. By doing experiments, they are not rejecting the possibility that living things mop up the products in natural environments. They would of course need to make the environment sterile, to avoid biological contamination. Trying to achieve abiogenesis in the lab is not a statement about the competitiveness of the organisms they fail to make. It might, as I have said, prove impossible to do, even if it ‘really happened’. But the reason is not nonlin-entropy.
If they formed amino acids, there was entropy decrease. There is less entropy in an amino acid than there is in ammonia/CO2. The molecules shed energy to get there. This is also why you get them on meteorites. Local decrease accompanied by wider increase is still thermodynamically spontaneous. It’s kind of a Law.
Tough to control for this, by sampling at (say) ocean vents miles down, and teeming with organic molecules. I’m doubtful anyone is even looking. You seem to think they should crawl out from the deep like Godzilla.
So, what if they ingest 100%? Or we can’t detect the remaining 0.1%? Another aspect I failed to mention: conditions are now much more oxidising than they were 4 billion years ago. If it needs a reducing atmosphere, this is again a rational reason we would not see it as a daily occurrence in nature.
Nonlin.org,
More ignorance. Abiogenesis does not require ‘spontaneous fluctuations’, and ‘equilibrium’ is not the sole alternative to them. Indeed, your view of ‘equilibrium’, the utter confusion between thermodynamic and other kinds of equilibrium, is the most embarrassing thing about this whole concoction. A high school howler of the first order.
As I already wrote, my dear illiterate entertainment, if the pathways are different it doesn’t matter if you want to call them “reverse of each other,” there’s no entropy problem. You lose no matter what you want to call them.
Of course, which is why the pathways have to be different.
Merely calling something “reverse” doesn’t make entropy forbid abiogenesis. What matters is that the pathways from molecules to life are not the same pathways as those going from life to molecules. Both processes happen all the time. So, if you want to call molecules-to-life “reverse-decay”, and it happens all around you, then entropy doesn’t forbid it. Guess what? It happens all around you!
It’s very amusing that you’d believe that just by calling something “reverse,” it magically becomes a reality that goes whatever way you want. Sorry, but you’re not a magical being in the sky who can change reality by redefining words. You’re just an obtuse and illiterate kindergartener.
I am looking for some clarification here.
Under nonlin-physics, bringing a 3quanta-4particle system into thermal contact with a 2quanta-1particle system will lead to a 5quanta-5particle system.
Measuring the nonlin-entropy before and after, before it is Kb.log(4) + 0
After, it is Kb.log(1) = 0.
So, in nonlin physics, (A) this is an example of entropy decreasing spontaneously, in an isolated system.
(B) from the perspective of the 4 particle system, it is receiving energy, and its entropy is going down; is this the sort of example nonlin was looking for? If so, why preface the example with a “having said that,”
Now, nonlin maintains that local entropy cannot decrease spontaneously, and EVERYBODY maintains that entropy cannot decrease in an isolated system. Your example is a breach of the 2LoT, according to you, nonlin.
In regular TD, the entropy has increased from Kb.log(20) to Kb.log(126)[I think…]
And to answer your question, I have learned that there are certain systems wherein adding energy leads to a reduction in entropy. Such systems have negative temperature (by definition: dS = dQ/T), like I said. They are rather esoteric, and have no application at all to biology, hence my caveat :
But if you have a biology-relevant example where adding temperature leads to a reduction in entropy, without violating 2LoT, provide it now.
Well, if you cannot count microstates properly, very few of them are going to make any sense at all. Educate yourself.
Yes, we do. What goes in, must come out.
Not at all. When you reverse one of your actions, you don’t walk back cartoon style.
So you do NOT have ONE example of organism without homeostasis. Then ‘primordial’ it is.
Crystallization does happen. That doesn’t make it trivial. Crystals have been rare, therefore precious. And anyway, we’re comparing entropy increase vs decrease. So, smashing a crystal is always WAY EASIER than making one. QED.
Maybe not your claim. Someone’s. The rest makes no sense. WTF does “life works by following thermodynamic gradients” even mean?
You’re not the product of abiogenesis. So cut the crap.
The inputs of what? To “abiogenese” a cell, you start with the composition of a cell. That’s CHNOPS . Same for a cake, a car, or what have you. Simple.
What “local” entropy decreases? If any of that kinetic energy goes into chemical energy and lowers local entropy, it MUST BE that not all 100% was dispersed. Example?
Of course they do. Else they wouldn’t bother knowing “living things mop up the products”. And sterile labs is what I said. No one cares about “in nature”. Just do abiogenesis whichever way you can.
That can’t be. You invoke a “materialistic miracle” , see par 9. And the reason is EXACTLY what I outline here.
You missed the point.
“I can’t test” is a bullshit excuse. There’s many ways around your mental barrier.
That’s what labs are for. Look, if there were something there, we would know by now. Same with Miller-Urey. No real follow-up because there’s nothing there.
By definition, nothing happens in equilibrium. Other than entropy increasing decay. And when equilibrium forbids fluctuations (as it does), abiogenesis is in doodoo. Name ONE alternative!
More precisely, there’s only one path and no return. From low to high entropy.
But then again, if the quanta is kinetic energy (heat) or electricity, bringing those particles together is not spontaneous (requires external energy). So that explains the entropy reduction. Problem solved.
No, it isn’t as I just explained. Haha. Missed again. So professor there (MIT no less) ignores the effort to bring those two systems together because he switches mentally from macro to micro without adjusting, and regurgitators blindly repeat what they were told. But science is advanced by people that think, not by regurgitators.
Not sure what you’re getting at. Which microstates are consistent with the macrostate? As I explained not all that are thought so. The energy is always distribute evenly at equilibrium. Remember the hot iron rod? What’s your concern with “biology-relevant”? You know that all organisms have an optimal temperature (or range given homeostasis), right?
Don’t be silly, you wouldn’t be sitting there, let alone writing anything, if there was only one pathway. There’s plenty of pathways, all running according to the laws of thermodynamics. Some go through an enormous variety of life forms, some through crystals, some through storms, some through spectacular cosmological events, etc. For all we know, there might be plenty going through abiogenesis, which might leave us in the years to come with a plethora of possible ways life could have started in out tiny planet.
For as long as there’s energy imbalances, pathways will abound, pathways will diversify, and have all kinds of interesting phenomena fuelled on. All despite Nonlin’s imaginary power to change everything by merely calling them “reverse.”
Nice display of illiteracy though. You’ll do anything, even ridiculing your reading abilities, before admitting to be mistaken even if a tiny bit, even when your misunderstandings are clearly contradictory to each other and to the reality that surrounds you.
Thanks again for the entertainment.
This is so much fun.
The person who wrote:
Is the very same person who wrote:
Poor Nonlin cannot even agree with herself.
🤣
nonlin, you cannot go two paragraphs without contradicting yourself. First up, your argument against abiogenesis is that local entropy decreases are not possible, even with the “application” of “external” energy. You have just proven yourself wrong!
But before you start spluttering about the meaning of “spontaneous”, you made a mistake: you noted that the quanta in the 2quanta-one particle system could be kinetic energy. In which case that particle could spontaneously bump into the 4-particle system.
Ooops.
Not to worry, you are still wrong about what can happen in an isolated system: EVERYBODY agrees that entropy cannot decrease. So we are back with the point that your system for counting microstates (count only the most even distribution) leads to blatant contraventions of 2LoT. The reason is really simple: you are counting wrong. Quelle surprise.
You are welcome to try and give your best example where adding heat to a system reduces its entropy; just remember, no negative temperatures, okay?
No, we don’t. eg: My composition is not necessarily that of the first replicator, or its immediate precursor state. It’s not even the composition of my last few meals, even though that’s what I’m ‘made of’.
We aren’t talking of my actions, but of chemistry. You can burn hydrogen to make water. You can split water to separate the hydrogen and oxygen. Only a chemical and physical ignoramus would regard the processes as thermodynamic ‘reversals’ of each other, one increasing the other decreasing entropy overall. They both result in entropy increase.
This is just idiotic. You can’t determine what is ‘primordial’ by looking at modern things.
Bollocks. There are crystals on my kitchen shelf, on the beach, in my garden wall. They are neither rare nor precious.
Urgh. Your ignorance is showing again. You really don’t understand entropy. If you want to smash a crystal, you have to put some effort in.
If you need lessons in the energetics of living organisms, you aren’t up to critiquing the subject.
I am an example of an organism which can decay. So now you introduce a special kind of decay – nonlin-decay – that only applies to the initial product of abiogenesis. It’s that which is thermodynamically ‘reversed’ on abiogenesis, not any other example of decay I can come up with? Truly, this is a mess.
Not solely. Nonetheless, it is ludicrous to argue that the precise proportions of CHNOPS in a cell at the moment it commences decay must be represented in those precise proportions during the ‘construction’ of that cell. There is no cell on earth that was composed in such a way. QED, as you might say.
In the example I just gave, after coming to rest the kinetic energy was ‘100%’ dispersed from the rock in which it resided. I know you struggle a bit with ‘100%’ as a concept. It still has mass-energy, and thermal if it has a temperature, but the kinetic energy is dispersed. But entropy has decreased locally, simply because that energy is no longer available for work. Its work is done.
Your challenge appeared to be why we don’t see abiogenesis popping up in nature. That is, after all, where we’d expect it to happen, if anywhere. Its failure to happen in labs is much easier to account for, if that’s where you’re now retreating: we haven’t found the right conditions.
No I don’t. It’s simply that not everything that is possible can be reproduced in a lab. Experiments can fail for many reasons, without such failure ‘proving’ that the phenomena do not happen. I’m surprised they didn’t teach you that in Logic 101, under ‘proving the negative’.
Nope. You did.
Practical difficulties are real. “If abiogenesis were true it would be easy to detect” is not defensible as a syllogism, given the obvious possibility that it could happen and be hard to detect.
Of course! A handful of labs over the course of a few dozen years has not provided the answer. That proves there isn’t one! That’s logic, that is!
Miller-Urey was not an attempt to ‘create life’. It did demonstrate the thermodynamic favourability of amino acid formation in plausible prebiotic conditions. Similar experiments have produced sugars and nucleotide precursors. It’s certainly interesting.
Separating out this bit, since it is a masterclass in Getting Entropy Wrong:
Not in thermodynamic equilibrium. But your attempt to cast all equilibria as thermodynamic ones is laughable.
Ouch. That’s not an equilibrium situation.
No it doesn’t! God, you really are making a mess of this. ‘Fluctuations’ don’t only happen while entropy is increasing.
Allan Miller,
Oops! I could have expressed that better…
In other news, we had close to 10 inches of snow yesterday. Several cities were buried in “rare” and “precious” snow crystals. I wondered if my neighbours would buy my snow, being so rare and precious. That way I’d gotten the snow around my house shovelled and be paid for it (!!!!). But they seemed uninterested.
Today the temperature is fluctuating, something that shouldn’t happen because the planet is in “dynamic equilibrium.” So, the snow crystals are melting and the resulting water flowing away (noooooohhh!!! my preciouuuuuus!!!!).
I keep telling the snow crystals that they cannot go in “reverse” from snow-covered-to-snow-free, because entropy “forbids reversals,” to no avail. The snow refuses to obey Nonlin’s laws of thermodynamics and is leaving the place through a path that doesn’t really look like the reverse of the path by which it covered the place.
Given its rarity and preciousness, besides the “dynamic equilibrium” of the planet, I doubt we’ll have any more snow ever again.
Well, I did first learn about thermodynamics from Flanders and Swann. I was 11. I subsequently got a more formal education, learning about why “heat cannot of itself pass from one body to a hotter body”
OTOH, Mung may not have bothered to click through on my YouTube link…
Allan Miller,
I’ve been biting my tongue. 😀
Mmmm. The entire sentence is redundant, for the point I was making, in any case! I suspect that what nonlin is getting at is, if the system does work that lowers entropy locally, it can’t be true that all the energy ‘disperses’. Some must go to cause the local entropy decrease, rather than being lost. But I think it still ‘disperses’ in order to get there. I’m prepared to be proved wrong.
It’s the teacher in you. As said in certain computer book collection: “the redundancy is intentional and important.”
Comment was restricted to abiogenesis. There are no alternative paths from atoms to cell. That’s why Miller-Urey got so excited. They dreamed of reversing the decay path.
Delirium?
First case: “if abiogenesis were true”. Second case: “abiogenesis is not true”. Pretty elementary.
Are you misquoting out of ignorance or willfully? Of course local entropy can decrease.
That doesn’t mean it transfers and most certainly doesn’t mean they combine to form a new system. And let’s not forget the assumption is “no energy losses”. You too are porting from macro to micro without any adjustments. And more to the point, you’re grasping at straws.
What do you mean?!? “Entropy cannot decrease”? EVER? Under any circumstance?!? I think EVERYBODY DIS-agrees with your ignorant claim.
On another note, I know I’m disagreeing with the formula, but for good reasons:
1. No microstate fluctuations have EVER been observed in thermodynamic equilibrium
2. Consider a piston. If that gas ever goes back to the corner by itself, you got yourself a perpetual motion machine and that’s no good. Furthermore, we know it doesn’t [go by itself] for the simple reason it increasingly resists compression in real life.
3. Consider a cylinder with gas in a density gradient. That’s a valid microstate according to the current theory. But you know what? That macrostate doesn’t match the macrostate of the uniformly distributed gas because pressure is different. And you know where this cylinder lives? That’s right, it’s an atmospheric column. So this formula that disregards the macrostate MUST be wrong.