As discussed here extensively, nothing in “evolution” makes any sense: “natural selection, fitness, speciation, human evolution, gradualism, divergence of character, UCD, TOL, etc. etc.” Not one makes sense. Meanwhile, the “evolution” argument is just one big “affirms the consequent” logical fallacy, while Paley’s excellent argument has never been overturned, and an intuitive intelligent design detector can be used to easily disprove “evolution”. Is there a need for any more proofs? Not really. Are there any other proofs? You bet. Take entropy for instance…
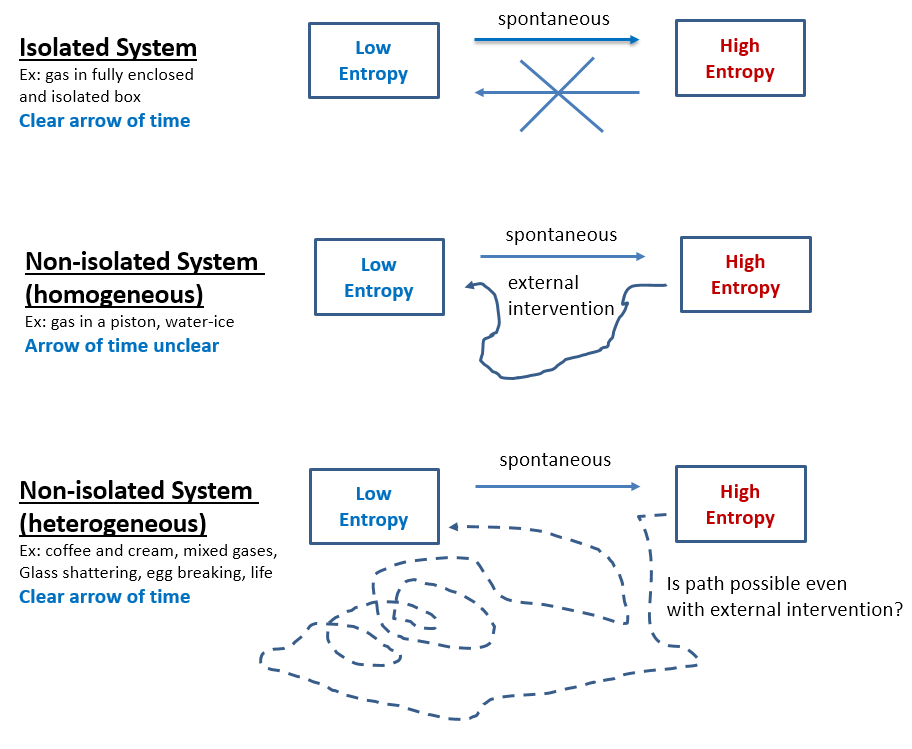
Figure 1
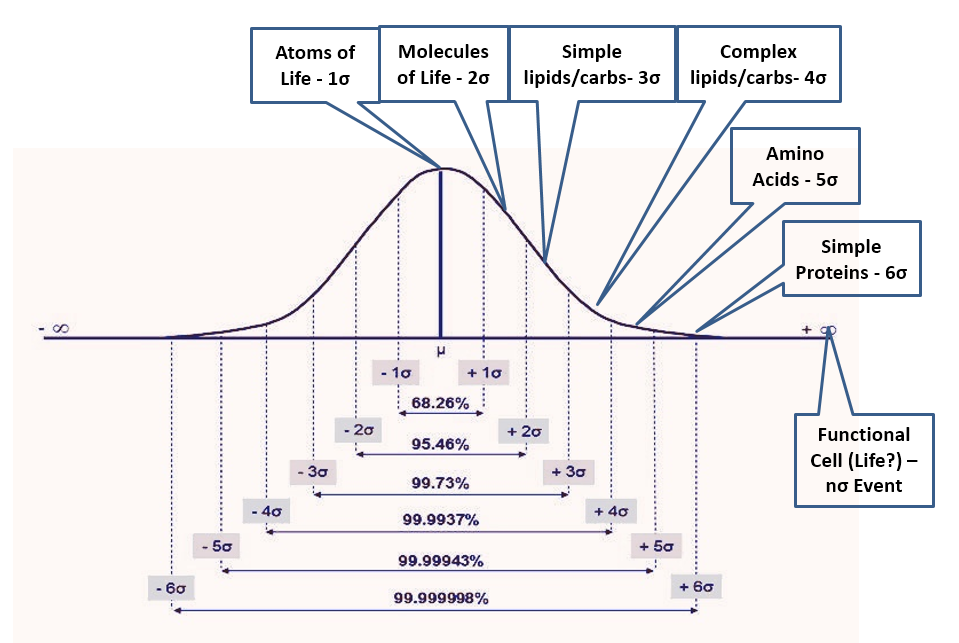
Figure 2
- Second Law of Thermodynamics shows that a spontaneous process cannot also revert spontaneously. This is because spontaneous processes always increase the system’s entropy. A uniform gas in a chamber will accumulate in a corner only with external intervention and spontaneous chemical reactions can only revert if external work energy is applied. Current models of entropy assume the gas particles in a chamber to be independent (sometimes represented as pebbles on a Go board) and explains their never observed convergence on one side of the chamber as only due to that particular microstate having a very low probability(*). However, gas particles always interact with each other (Brownian motion) while pebbles do not. Thus, a reliable way to know that entropy of a system increases is if work energy could be obtained when transitioning from the low to the high entropy state while energy is always required for the reverse process.
- Total entropy of an isolated system can never decrease. Entropy is currently assumed just a statistical law. Thus, if N molecules are in an isolated system (box), the number of microstates associated with j of them being in one half while N-j being in the other half is Ω = N! / (j!*(N-j)!). If N is small, fluctuations seem possible, but before N increases to anything measurable, the probability of fluctuations rapidly decreases to nil. Furthermore, even these theoretical fluctuations, as improbable as they are, might be impossible since the statistical view does not account for molecular interaction observed as Brownian motion and as gas resistance to compression and expansion. Better fundamentals or statistics, either way entropy will never decrease spontaneously in an observable system (Fig 1.a).
- Decreasing entropy is not the reverse process of entropy increasing. That is why a broken egg coming together is easily identified as unreal and a reversed movie of its real shattering. The known laws of physics are the same forward and backward (time-reversal invariance), therefore the reverse shattering process of an egg would not violate any law, but only because these laws are always idealized. Supposedly, if just the right forces are applied to the broken pieces, the egg will come together. In reality this is impossible, and not because the unbroken egg is a highly unlikely microstate, but because entropy increase is not directly reversible even in non-isolated systems. This irreversibility holds for all heterogeneous systems, including life which is perhaps the most heterogeneous system of all. Entropy increase is directly reversible only for homogeneous systems and only if in a defined space. For instance, an expanding gas in an ideal piston creates a force that, when reversed, compresses the gas back into its original state. However, a solid cube of ice can be easily melted by increasing the temperature, but the original ice cube will not reconstitute by lowering the temperature, hence this process too is irreversible despite the cube of ice being homogeneous (Fig 1.b). As far as heterogeneous systems, even separating two mixed gases is way different than the original mixing process, hence mixing is irreversible (Fig 1.c). Entropy decrease is not only different, but also much more complex than entropy increase which is usually spontaneous. Abiogenesis is the entropy-lowering reverse of the biologic decay process, and therefore – if at all feasible – much more complex than adding chemicals and energies.
- Once in equilibrium, a “primordial soup” does not change spontaneously. Life is metastable – it requires certain forms of energy to sustain and spontaneously decays when it no longer receives that energy as well as after the end of the normal lifespan of the organism. It was hypothesized that random fluctuations can spontaneously create compounds and structures given enough time. Abiogenesis, as a reverse-decay process, cannot simply be an outcome of Brownian motion of the chemicals mix because a perpetual motion machine powered by decay and abiogenesis cycles would violate the ‘conservation of energy’ principle. Experimentally, one can confirm that chemical blends in static equilibrium never transition spontaneously into a different equilibrium state (this includes oscillating reactions after the settlement period).
- A “primordial soup” cannot generate life even if energy is applied. It was hypothesized that abiogenesis can be a product of tidal pools, deep sea hydrothermal vents, and the undersurface of ice caps where persistent and abundant energy is available in the form of thermal and electrochemical gradients. Indeed, energy can throw systems off balance and create all kind of chemical compounds and physical structures. However, as the energy applied increases, a complexity limit and hence a dynamic equilibrium is reached where molecule destruction offsets their creation and, if even more energy is applied, molecule destruction dominates, eventually leaving the experimenter with gunk and none of the desired molecules. Miller–Urey and subsequent experiments were not ended because they reached their goal – life – nor because they ran out of energy and materials, but because they reached this dynamic equilibrium, and by adding more of anything would have left them with fewer of the targeted compounds. The amino acids obtained were not the end product but the intermediate between the original molecules and the useless gunk that was the product of the Maillard reaction caused by the energy applied to the system. More complex molecules (and maybe life itself one day) can be created by intelligent designers adding targeted compounds and energies. Then “why can’t natural processes somewhere somehow just mimic the intelligent designer in this vast and almost timeless universe?” The better question is: “why insist on natural processes when the model to be mimicked is that of the intelligent designer?”
- If natural processes were capable of generating life, the environment would be full of intermediate bio-compounds. Life is so complex that laboratories have no hope of replicating it in the foreseeable future. However, if abiogenesis were an outcome of natural processes, the cell structure would be produced only from subsystems and complex biomolecules that in turn would depend on simpler molecules down to H-C-O-N, the atoms of life. A “primordial soup” capable of generating life, thus must contain all intermediate compounds from the atoms of life to the most complex biomolecules and subsystems in an ever-decreasing ratio as complexity increases. Not knowing anything about how this process would work (or even if possible), the most reasonable assumption is a normal distribution of outcomes with life being an n-sigma event (with n unknown) while the availability of the atoms of life being a 1-sigma event and anything else falling in between (Fig 2). Many x-sigma events would be required for each (x+1)-sigma event, with a good first approximation given by the normal density function. Thus, the 2-sigma event could be the basic molecules of life (water, methane, etc.), and we would expect only one of these events for every seven of the 1-sigma events. This approximation would further yield (in one scenario) 1/7 fewer molecules of life than atoms of life, 1/17 fewer simple lipids and carbohydrates molecules (3-sigma) than of molecules of life, 1/43 fewer complex lipids and carbohydrates (4-sigma) than 3-sigma events, 1/110 fewer amino acids (5-sigma) than 4-sigma, 1/291 fewer simple proteins (6-sigma) than 5-sigma, 1/771 fewer complex proteins (7-sigma) than 6-sigma and then – rule of thumb – 1/1600 (8-sigma), 1/3800 (9), 1/9100 (10), 1/22k (11), 1/52k (12), 1/126k (13), etc. fewer of each additional sigma event than previous event where 8+sigma being (this scenario) nucleic acids, short chains, long chains, organelle subsystems, organelles, other critical cell components and finally the fully functional biologic cell – the n-sigma event which is not quite life but good enough for this analysis. Then how can we test this?
- Apart from life itself, the complex molecules of life are nowhere to be found in the universe. To test the ‘natural processes’ hypothesis of abiogenesis, one must observe the intermediate components of life in nature and in the ratios estimated above (or from another reasonable estimate). In addition, one must observe the spontaneous transitions (aided by energy) from simple to complex even if not all transitions are observed at once. Earth is “polluted” with life down to the deepest ocean trenches, therefore the first focus is the extraterrestrial space where, too bad, the largest confirmed interstellar molecules have a maximum of 13 atoms (apart from C60/C70 fullerene). Back on earth we see all intermediate components, but only within life itself. Outside of the cells, aside from the simplest biomolecules, we only see products of decomposition that are never in the ratios associated with abiogenesis, meaning we never see increasing molecule complexity in decreasing ratios resembling anything reasonably expected. Abiogenesis is not happening due to the irreversibility of the entropy increase and for the same reason egg breaking, butter melting, gas mixing, etc. are not reversible processes. Humans can only create a few of the complex molecules, although most always aided by life itself, and even then the power of synthetic biology is severely restricted. The more complex, the harder these molecules are to obtain and the faster they decay instead of spontaneously combining with one another to form even more complex compounds and ultimately life.
- Miller–Urey style abiogenesis experiments are ill conceived, hence doomed from the beginning. To be more specific, they are only good for PR (public relations) given the irrelevant “organic compounds” created that raise the hopes of the believers. Trying to obtain an automobile from scratch by mixing chemicals and energy, qualifies the person attempting as delusional and the one selling such vision as charlatan. So why would those attempting the same with life – which is infinitely more complex than an automobile – not also be labeled charlatans and delusional? Abiogenesis experiments belong to the Reverse Engineering category of processes and, when done right, they are very different than Miller–Urey. Their starting point is never some “primordial soup”, but the most advanced compounds available, preferably already organized in working subsystems. Swapping organelles or parts within organelles, exposing organisms to various environments, attempting to revive dead organisms, substituting engineered subsystems and so on are part of the hard work with long tradition and already being done in medicine and many industries for other purposes than to prove abiogenesis. If and when someone will be able to reverse the decaying and dying processes, we will know that abiogenesis is possible as an act of Intelligent Design creation. To confirm abiogenesis as an “unguided process” we would have to observe reverse-decay and reverse-dying processes happening in nature, not in a lab. Yet 2nd law proves this impossible.
- Is abiogenesis not feasible because it was a unique event? If true, abiogenesis would be a “materialistic miracle” and furthermore not just one, but a long series of “materialistic miracles” since a long series of – so far unknown – events are needed to get from atoms to the simplest organism. Yet one of the tenets of materialism is “no miracles” showing the inconsistency of the materialistic “unique event” assertion. And of course, physics and chemistry transformations are never unique. And even if entropy allowed for abiogenesis, the laws of life do not follow from any priors (physics, chemistry, mathematics). Life has a drive to survive and leave off-springs which entails harm avoidance, immune system, metabolism, food seeking, homeostasis, growth, reproduction, and body structure. Without these, any cell would start decaying the instant it was formed as in fact it does as soon as it no longer is alive. Despite having lasted almost since the formation of The Earth, life is metastable – one knock and it dies and then decays. This is unlike other negative entropy machines that can be restored (rebuilding proportional with the damage).
- Other considerations.
- “Dissipation-driven adaptation of matter” (J. England, MIT) claims that life is inevitable because life “absorbs and dissipates more energy from external sources” leading to faster entropy increase. However, there is no law that entropy has to increase faster. In addition, most of the entropy in the universe is captured by black holes with life having a nil contribution to that entropy.
- Some claim they have obtained “protocells” that seem to mimic real cells at least in part. However, “protocells” are to biological cells as fool’s gold is to real gold.
- “Kolmogorov complexity is lowest at low and high entropy and high in the middle hence life is supposedly inevitable (S. Carroll)”. However, life is not complexity. Life is much more than snowflakes, vortices and chemical reactions (candle burning). And most certainly, life is not the complex swirls of cream mixing into coffee on a journey from low entropy to high entropy (both having low complexity). In addition, unless very specific external action continues to be applied to maintain those patterns, they soon disappear like in sand dunes exposed to shifting winds. The patterns therefore do no “arise”, but are created by an external force.
- “Gradients of energy in deep vents are responsible for abiogenesis”. But all organisms from these exotic places are very similar to any other ones found elsewhere, hence all likely have the same origin. In addition, no free floating organic compounds (aside from decay byproducts) have been found there to suggest ongoing abiogenesis. And, aside from the simplest molecules, no spontaneous transitions from x-sigma to (x+1)-sigma bio complexity has ever been observed around these deep vents either.
- Of course life does not violate 2nd Organisms do conform to 2nd law when they decay as soon as they die. In addition, as observed by Erwin Schrödinger, “the increase in entropy from turning our low-entropy food into our high-entropy waste is greater than the local decrease in entropy from making the well-ordered structures within our bodies”. Nothing special so far – a refrigerator does the same: creates a zone of low-entropy while the entropy of the whole system increases and for as long as it’s fed energy.
- Randomness can theoretically account for any bizarre occurrences including Paley’s watch and F. Hoyle’s 747 in baby steps if enough time is given. But no such event was ever observed. In addition, breaking down the unattainable complex system into a combination of simpler components, each with higher probability of occurrence makes it no easier as the probabilities of all subsystem have to be multiplied to get back to the complex final assembly.
- Some claim that life itself prevents abiogenesis by ingesting all intermediate molecules spontaneously formed, but this can be easily prevented in sterile labs. In addition, all complex intermediate molecules observed outside of cells are due to decomposition, not abiogenesis.
- “Evolution” corollary number 1. If abiogenesis is impossible as an undirected, natural process, then whoever is responsible for abiogenesis is also responsible for the biologic landscape past and present, therefore “evolution” is also impossible as an undirected, natural process.
- “Evolution” corollary number 2. It is easy to verify that nothing ever “evolves” in the nonliving nature. Life is said to be “just chemistry”. These two combine to: nothing “evolves” in the living either. Solar systems, geographical features, fluid eddies, chemistry, snow flakes, etc. all go through their life cycles, and all are different from each other, but the life cycles of the newer entities are no more “evolved” than the life cycles of the ancient ones.
- “Evolution” corollary number 3. Presumably, “evolution” has not ended. And if ongoing, then one must see the normal distribution of the different transitioning organisms (the intermediary), just as we would see if abiogenesis were true. If humans evolved from monkeys and “evolution” is ongoing, then humans must still be in transition especially since the human population is one of the largest of all mammals and, the more individuals, the more “evolving” opportunities. The older Darwinists replied with a hierarchy of races. But that reply is not only fashionably repugnant, but also false and, amazingly, contrary to [at least] the Abrahamic religions that have always known better.
- In conclusion, abiogenesis is nothing more than the decay process running backwards, therefore easily visualized, yet impossible according to the second law of thermodynamics. In other words, “evolution” is nothing more than imagination run wild. Expecting abiogenesis to be within reach if only the proper forces and chemical compounds were added is as wrong as expecting the broken egg to come back together if only the proper sequence of forces were applied to the broken pieces.
Summary:
- A spontaneous process cannot revert spontaneously.
- Mixtures will never ever spontaneously separate per second law.
- Decreasing entropy is not the reverse process of entropy increasing and also much more complex.
- Once in equilibrium, a “primordial soup” does not change spontaneously.
- A “primordial soup” cannot generate life even if energy is applied due to dynamic equilibrium.
- If natural processes were capable of generating life, the environment would be full of intermediate bio-compounds.
- Apart from life itself, the complex molecules of life are nowhere to be found in the universe.
- Abiogenesis experiments belong to the Reverse Engineering category of processes.
- Miller–Urey style abiogenesis experiments are ill conceived, hence doomed from the beginning.
- Abiogenesis unique event conflicts with the “no miracles” clause of materialism.
- Even if entropy allowed abiogenesis, the laws of life do not follow from any priors (physics, chemistry, mathematics).
- “Evolution” corollary number 1 – no abiogenesis, no “evolution”.
- “Evolution” corollary number 2 – no “evolution” in the inert and “life just chemistry”, then no “evolution” in the living.
- “Evolution” corollary number 3 – no intermediate “evolving” entities, no “evolution”.
- Being a decay process running backwards, abiogenesis is as impossible as a broken egg being reconstituted by the “proper sequence of forces”. “Evolution” is also nothing more than imagination run wild.
(*)R. Penrose “The Emperor’s new mind”; PBS SpaceTime “The Misunderstood Nature of Entropy”; Sean Carroll “From Eternity to Here”, etc.
Links:
Abiogenesis: The Faith and the Facts
James Tour: The Mystery of the Origin of Life
Chirality, Maillard – caramelization, characterize the structure at every step:
https://creation.com/why-the-miller-urey-research-argues-against-abiogenesis
https://evolutionnews.org/2014/06/squeezing_the_l/
https://www.ncbi.nlm.nih.gov/pubmed/21422282
Entropy of a box of molecules
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2253472/ https://en.wikipedia.org/wiki/Normal_distribution#Cumulative_distribution_function
http://physics.bu.edu/~redner/211-sp06/class-engines/class25_secondlaw.html
https://www.quora.com/How-quickly-is-the-entropy-of-the-sun-changing
https://www.thoughtco.com/how-many-atoms-in-human-cell-603882
https://www.amazon.com/Mysteries-Modern-Physics-Sean-Carroll/dp/1598038699
https://en.wikipedia.org/wiki/File:Elements_abundance-bars.svg – abundance in the solar system
https://en.wikipedia.org/wiki/Miller%E2%80%93Urey_experiment
https://en.wikipedia.org/wiki/Proteinogenic_amino_acid
https://en.wikipedia.org/wiki/Artificial_gene_synthesis
https://www.scientificamerican.com/article/a-new-physics-theory-of-life/
https://en.wikipedia.org/wiki/Dissipative_system
https://pubs.usgs.gov/gip/dynamic/exploring.html

Really? It looks much more as if this is about your hurt ego.
So massive that it happens all the time all around you. Lots and lots and lots of new life forms, from molecules, all the time. And not all are unicellular, mind you. So you don’t have the excuse that you never see such a thing happening.
It looks very possible, even easy, in the naturally-directed scenario that we experience all the time.
You deny your own fig 1 Nonlin. It shows different paths in one direction compared to the other, yet you keep saying that the paths are the very same in reverse.
🤣🤣😂😂🤣😂
DNA_Jock,
IKR? Well-known fact. You can’t cool things down without adding energy …
I will never understand why you call yourself a monkey, but let’s see that “point” of yours:
This is called a fallacy of composition. Look it up.
Let’s make a deal, remove your brain and let’s see how much logic you can perform after such “material” destruction. You might “prove” your “point” though, I doubt there would be too much of a difference.
Thanks again for the entertainment.
Only option 2 (I repeat for the reading impaired).
This has been discussed. Those not reading impaired know.
No problem. Here:
https://en.wikipedia.org/wiki/Collagen_helix
Your turn.
I always give due credit 🙂 And it’s just a convention. So no biggie.
Your mistake is in considering the incorrect initial state and the size of the system: https://en.wikipedia.org/wiki/Spontaneous_process
When using the entropy change of a process to assess spontaneity, it is important to carefully consider the definition of the system and surroundings. The second law of thermodynamics states that a process involving an isolated system will be spontaneous if the entropy of the system increases over time. For open or closed systems, however, the statement must be modified to say that the total entropy of the combined system and surroundings must increase,
It’s part of the process, not “prior history”.
Again, you’re disregarding part of the process. The reaction doesn’t start from the disequilibrium phase.
Watching half movies again? No wonder you keep losing the plot. Now here’s the FULL scenario: start with everything in thermal equilibrium at room temperature. Now cool without adding energy. Can you?
Not without life as a prerequisite. But abiogenesis lacks this prerequisite.
Not me. I have yet to see an abiogenesis scenario (from PROPONENTS) that doesn’t retrace decay in reverse. I’ll even look at sci-fi.
Only as soon as someone can prove “emergence”: https://en.wikipedia.org/wiki/Emergence
Btw, how many sheep does it take to “compose” an Einstein? 100? 10000? 1000000?
You can destroy all life and start over with abiogenesis. Guess what? Logic will still be there and identical to our logic.
Btw, I’m genuinely impressed, little one. Good job! More thinking and less flinging.
It doesn’t matter, if it’s not possible because of entropy, then it’s not possible because of entropy regardless of whether there’s life catalyzing it’s synthesis or not.
Are you saying that the person writing mindlessly that abiogenesis would be reverse-decay wasn’t you? Some scientist working on abiogenesis possessed you and started writing mindlessly that abiogenesis would have to be decay in reverse? Holy crap!!! How can you possibly break your records time and again!!! I’m truly impressed!
That’s quite another record for you. It’s not necessary to prove emergence for a fallacy of composition to remain a fallacy of composition. It’s obvious that the parts don’t need to be able to do what the whole does in order for the whole to function. Computers are not made of microscopic computers. Cars are not made of microcars.
I don’t know what Einstein’s mother ate when she was expecting, nor how much. It’s still amazing that you think that Einstein was built from microEinsteins though.
Well, all you have to do is remove your brain and prove it. Given your “emergence” lame excuse above, I think you’d win this one.
Thanks again for the entertainment. Absolutely impressive. Now scientists possess you and computers are built with microcomputers, which are built with nanocomputers, which are built with femptocomputers, etc. It’s turtles all the way down for you I guess. Now I know why you’ve got so angry when I said you were built from molecules. You thought you were built from micrononlins, made from nanononlins, made of femptononlins …
Why, yes, that is what I meant. I used the word “solidification” to distinguish your (1) “evaporation crystallization” from your (2) “cooling crystallization”. But I am still somewhat surprised to learn that “cooling crystallization” requires a fridge. So ~4.5 billion years ago, the earth was stuffed into a very large fridge. Truly, nonlin physics is amazing.
Not for a Yorkshire henhouse, it hasn’t. But I will take your refusal to answer as an admission that you are incapable of calculating the change in configurational entropy.
The entropy of this eggshell has decreased, by the way. Do you understand why?>
Nonlin.org,
Since the point was about breaking eggs, not chemically altering them, that’s irrelevant.
Sorry, what system we talkin’ ’bout now? Eggs? Crystals? Your fridge? The prebiotic world?
“Q1: calculate the entropy change when a 1kg weight is allowed to fall from a height of 10m”
Nonlin: “sorry, I need to know how it got there”.
Haha.
Sounds like you’re talking yourself out of your entire thesis. If you need to trace back in time, and include prior history, you really aren’t in a position to categorically declare that ‘entropy forbids abiogenesis’.
Also, it would be a revelation to generations of chemists that quoted reaction ΔG’s are wrong because they don’t include the reactions before. Where do you stop with this ‘backward extrapolation’?
OK, thermal equilibrium, room temperature. Got it. But my house gets cold at night. I don’t add energy to make that happen. Indeed, I (or rather, my wife; it’s a constant bone of contention) have to add energy to stop it happening. Likewise the equilibration of my fridge: I have to add energy to stop it. It doesn’t work when it’s not plugged in.
If the fridge is on – consuming energy to oppose equilibration – it marginally slows down the night-time cooling of the room. I’m using the heat taken out of the fridge interior to warm the room, as well as a bit of motor energy. Of course, the interior cools. But that’s not the FULL scenario, is it?
Nonlin,
I might not be in the forum for a while, lots of end-of-term work. So, here’s my holiday gift for you:
Gift for Nonlin.
Enjoy!
Living systems are very adept at energy conversion and life can grow and maintain itself without the need for intricate thermoregulation. The success of plants demonstrates that complex temperature control is not a necessity for life to thrive.
But if life is to develop consciousness, tightly controlled thermoregulation is vital. If an organism is to achieve consciousness it must allocate a high percentage of its energy conversion to do so. Metabolism adds to the growth and maintenance of an organism, but the processes concerned with consciousness take energy away from growth processes.
Life evolves by working against the natural disorganising principles that are measured as entropy. Entropy doesn’t forbid evolution, but life must evolve by overcoming the natural forces which tend to increase entropy. It does this in multiple, well-coordinated ways.
Of course it is necessary. Because your false assumption is that you know what the whole is composed of. Only once we agree on what the whole is actually composed of, only then you can speak of a fallacy of composition.
The rest doesn’t make any sense. Why do you have to regress to your mean so fast? Remember “more thinking”?
One guy talks about his 4.5 bya experiment. Because why would he run the experiment here and now? Another writes War and Peace – the comical version. Just to not concede the lost point. Pretty funny I guess.
From what state to what state exactly? I’d like to see your detailed estimate but, as a guru-in-training once said, “I will take your refusal to answer as an admission that you are incapable of calculating the change in configurational entropy.”
It’s quite simple and amazing that such an outstanding chemist, pardon me, bio-, doesn’t get it. The point is that protein (mostly collagen) holds the eggshell together – which it does even when CaCo3 is removed as shown. And being the long molecules they are, some will most certainly break when the intact eggshell is broken. QED.
Meanwhile, you turned your past due proof into a “you first” game and still didn’t answer. And will never do because obviously YOU CAN’T possibly provide the promised proof. Haha.
Not the same. If the ball sits there for ever until a lever is pulled, it’s in equilibrium. If it just bounced and is at that infinitesimal point where speed is zero, that’s disequilibrium.
Please correct me if I’m wrong 🙂 , but I believe entropy has always been calculated equilibrium-to-equilibrium. It’s already hard enough as it is. Will Boltzmann’s even apply to potential energy? Show how!
No, not the whole prior history. Just equilibrium to equilibrium as explained. Breaking down huge problems into small manageable pieces is SOP and I’m shocked (smelling salts please!) you actually don’t know this.
You already got the amino-acids, the vesicles, the basic molecules, and latest technology. And you know what? I’ll throw in all the bio-products you can get. And don’t even worry about abiogenesis. Just make some proteins, some DNA, etc. Just baby steps. But do it abiogenesis-style. Don’t use the living to do that for you (and no, PCR doesn’t count). And see how hard it is to lower entropy in heterogeneous systems. I’d say “go separate cream from coffee”, but who needs that? Whereas making proteins will be richly rewarded if you can.
Thanks. I hope this time you finally graduate pre-K 🙂 I know I did my best with you. Success!
Not directly, yes. But see corollaries 1-3.
Change in entropy of the eggshell from the start to the finish of the scenario described. I’m sorry, I thought that was obvious. You appeared to claim you knew the answer.
https://en.m.wikipedia.org/wiki/Phase_transition
Nonlin.org,
Another pretends he didn’t understand – or maybe he really didn’t – in order to cling to his wholly incorrect notions re: fridges and entropy.
Layering on the bluster doesn’t help you. Naturally, the first opportunity I gave to write down the reaction involved the 97% component, rather than the 3%. Now, regarding that 3%, you’re just guessing:
Prove it. I contend that the intermolecular forces – hydrogen bonds, van der Waals – will shear before any covalent bond is broken. They are much weaker. It is, as I rather cleverly pointed out, like expecting a chain to shear at a metal link, when furnished with a few intermittent paper ones, and tugged.
Wrong. The situations are identical.
Gladly. You Are Wrong.
That’s not true – Google is your friend – but also real-life entropy change is not constrained by what we can calculate.
Don’t you know anything about entropy beyond Boltzmann? Educate yourself. Potential energy is absolutely central to numerous treatments – not merely gravitational, but (relevant here) chemical.
So because of a perceived calculation problem, real systems cannot have continuous transitions, or be perpetually out of equilibrium? Ha and, indeed, ha. That’s rivers stuffed, then. And organisms.
Entropy isn’t a measure of ‘disorganisation’. This viewpoint informs no end of misunderstandings.
Entropy is a measure of the capacity to do work – it relates primarily to energy, and not to arrangements of matter per se. Life actually works with the 2nd Law, not against it.
We have been discussing the breaking of a generic egg. Which somehow turned into the breaking of an eggshell. Now you come up with a very complex scenario (do you live near that particular Yorkshire?) and make a specific claim. So go ahead and answer your own riddle. If you have something to say, that is. More importantly, what has that to do with “entropy forbids abiogenesis”? If NOTHING, then I’m not chasing your riddle.
Your answer was bullshit. You own a fridge because you cool with it. And guess what? It requires energy. The day and night cycle doesn’t always work. Plus you need to get up (oops, energy) to open the window. So more BS from you.
That may be. But it’s not ONE link vs ONE other. There are lots of loops and concentrated kinetic energies: https://pdb101.rcsb.org/motm/4#:~:text=Collagen is composed of three,acids forms this sturdy structure.
And we’re only talking about the 3% because of the chemical bonds artificial limitation. As far as entropy, it goes up if either inter- or intramolecular links are broken.
And you still haven’t proved that NONE of these chemical bonds is broken. You’re not even trying. That is your concession non-speech.
If so “central”, then is Boltzmann wrong to not include potential energy in his formula? Set him straight, will you? Can you?
Not at all what I said. Your initial claim was that local entropy decreases spontaneously. Which is false according to the general definition of “spontaneous”. Which you countered with the “chemist’s definition”. Which is not really true since that doesn’t describe something truly spontaneous, but rather something feasible such as taking a system from a metastable to a more stable condition.
And if entropy lowering is so easy as you claim, how come this challenge can’t be met even theoretically? Describe how you will approach this problem. Now that you got your honey all crystalized… Spontaneously… Allegedly… Haha.
Honestly, yes. But that doesn’t mean I’ve made false statements.
You were the one who proclaimed that entropy is a measure. You didn’t know what you were saying?
Oh my. If you want to have a conversation about thermodynamics you need to demonstrate that you’re competent. I’ve stated what i think, repeatedly.
You appear to be unable to grasp the statistical nature of thermodynamics. It has nothing to do with how you define hotter. I’ve never seen a thermodynamics text that depends on how you define hotter.
How do you define hotter and how is it relevant to thermodynamics, as in, what prevents something not as hotter from exchanging whatever you think is prevented from being exchanged?
If body x is this hot, then body y, which is less hot than body x, cannot possilbly transfer y to body x, because physical law z declares it is not just unlikely (not a matter of probibilty), but impossible!
Thank God for fools.
Entropy is a measure. If we could all agree on that, we may be able to make progress. What is entropy a measure of?
I didn’t say that entropy was a measure of disorganisation. I said that disorganising principles are measured as entropy. Take the phosphorylation of ADP. That is an organising principle whereas using ATP to produce energy is a disorganising principle. The fact that in these processes work is put in and work taken out surely satisfies your definition of entropy as a measure of the capacity to do work.
Now consider physical activity and mental activity. Both of these activities require energy input. During physical activity muscles will be built up. This requires the growth of new muscle cells. Whereas mental activity will use a lot of energy but by this means brain cells will not grow in the same way that muscles cells do with physical activity.
In fact it has been shown that brain cells can also grow with physical activity.
From here:
Physical activity strengthens the organism, mental activity weakens the organism. Sleep counteracts the weakening effect of mental activity. Just as ATP must first be built up in order for it to be once again broken down to provide energy for organisms to function, so bodies must be built up to allow for consciousness which uses up bodily energy. Phosphorylation is a preparation for energy use, the development of our bodies is a preparation for consciousness. The whole reflected in the parts. Life advances by building up in order to tear down for higher ends.
[Bold added for emphasis]
Nice display of “convenient” illiteracy. You “forgot” the examples following that quote:
Had you been able to understand that tiny bit, you would have saved this spectacular embarrassment. First for giving us such an obvious display of illiteracy, second because, despite your selective illiteracy, the part you quoted is enough to understand that we don’t need to know every part making a whole in order to understand that the parts don’t need to be able to do what the whole does in order for the whole to function. A single tire doesn’t do what a whole car does, and it doesn’t matter if I know every part of the car or not. This is so obvious that I’d be surprised were it not because you’re so determined to break all your incoherence records.
Of course not. Your incoherence is just like that. Incomprehensible even to yourself, which is why you argue against yourself time and again and you don’t notice.
Thanks as always for the entertainment.
Back in the day, when you were trolling Sal (and agreeing with me and keiths, heh) you used to state what you thought. Last couple of years, not so much. Seems like you’re just phoning it in these days.
That ‘appearance’ is the result of your not paying attention.
I define temperature as 1/(δS/δU), as does Schroeder. Here’s an introduction to the idea. Hence my original comment regarding temperature being a derived concept, whereas energy and entropy are fundamental. In that same comment, my reference to Arrhenius was an allusion to the experimental support for Maxwell-Boltzmann. I was (rather obviously) making fun of nonlin’s belief that there cannot be fluctuations at equilibrium. You may have missed this.
I remain unconvinced that you understand stat. mech. You had a great deal of difficult with this simple statement about probabilities:
I’m not the one saying – for example – “the mind is just the brain which is just molecules”. You are. So when I mock the materialist claim…
Some little monkey has a point: being just a collection of mindless chemicals, “evolutionists” cannot possibly think, let alone be logical which is obviously immaterial (logic that is).
…you accuse me of the fallacy of composition. Which doesn’t even apply given that said fallacy would be: “true for parts, then true for whole”. Which is clearly different than what I’m saying: “NOT true for parts, then NOT true for whole”. Hence my demand to prove “emergence”. Meaning if “NOT true for parts, then HOW would true for whole be possible?”
Don’t tell me you’re failing pre-K again? Better focus on your exams.
Before “making fun”, you should make an effort to understand the difference between ‘fluctuations’ and ‘variability’. Do you ever get embarrassed when you screw up?
No, I’m not.
Holy crap, did you ask your five-year-old neighbour to help you with that link?
That’s what you’re saying, true for the parts, true for the whole. Look:
Translation: Chemicals, the parts, are mindless / chemicals cannot think.
Translation: “Evolutionists”, the whole, cannot think / the whole is mindless.
This is pure gold Nonlin. You truly have outdone yourself this time. Let’s see what happens when we apply Nonlin’s logic:
Wow! Marvellous! It’s not a fallacy of composition any longer!
🤣
Hence? There’s a “hence”? Of course, of course. If a tire cannot do what a car does, how could the car do what it does at all?
🤣
Since I’m not the one desperately hoping that just changing “true” for “not true” will hide a fallacy of composition, you’re the one who should be worried about failing your pre-K again.
Your silly desperation is just beautiful Nonlin. Thanks again for the entertainment.
CharlieM,
Absolutely nothing is added by sticking ‘organising’ or ‘disorganising’ in there, simply to salvage your semantics. And those notions of ‘work put in’ and ‘work taken out’ are hoplessly muddled, too. In both the phosphorylation of ADP and the hydrolysis of ATP, work is done as a system moves to a lower-energy state – from lower to higher entropy. The system does work, and hence the capacity to do work is reduced.
Bloody ID thermodynamics! 🤣
Eta: without looking it up, say which is the more ‘organised’ state in the following two transitions:
a) from two amino acids to a dipeptide,
b) from two RNA monomers to a dinucleotide.
I know what I was saying, I simply have no idea what the fuck you are trying to say.
Haha. So it’s about intent now? Having created a cool zone, ice forms spontaneously – just as it does anywhere below freezing, however subzero conditions were arrived at. You don’t put energy in to make that happen. And my room only cools when I open a window? What a mangled heap of shite. This really isn’t the subject for you. But then, neither are statistics, chemistry, genetics … there is no beginning to your talents.
Not sure what the hell that’s supposed to mean. Chemical bonds are present in both the 97% and the 3% component.
I just cracked an egg on the side of a bowl (egg wash for a Beef Wellington, yum). The hard shell cracked, but the contents didn’t spill out because the membrane remained intact. I had to jab it with my finger. Now, you would have it that my fat finger has the capacity to break chemical bonds!
Like I say at the beginning of your posturing attempt to win a point, right answer wrong reason.
The burden of proof is on the proponent. Cracking eggs is a physical, not a chemical, change. Are textbooks wrong to frame it thus? Prove it.
Soz, he’s dead. But Boltzmann wasn’t the end of the entropy story. Potential energy is very much a part of it – see Gibbs, see Einstein.
‘True science’ depends upon using the colloquial meaning of a word? You’re a laugh a minute. But hey, argue semantics, it’s all you got.
It illustrates your basic problem in all subjects. You think yourself fit to teach chemists chemistry, geneticists genetics, statisticians statistics. But when you mangle the jargon, exposing yourself as a bit clueless on the topic, it’s they who are wrong.
(eta – I’d actually agree that ‘spontaneous’ is a poor choice of word where there is an activation energy barrier. However, that isn’t the case for many transitions, among which is crystallisation).
Allan Miller,
(Strictly, of course, entropy is only part of the capacity of a system to do work. However, it is a goddamned sight closer than using it as a measure of ‘order’, or ‘organisation’..).
Did you know that changing “true” for “not true” in a fallacy of composition, it stops being a fallacy of composition?
🤣
When discussing life these words (‘organising’ or ‘disorganising’) are central. Why are creatures called ‘organisms’? Organisms exist through a process of creating form by the constant gathering and scattering of material. The point is that organisms don’t just reduce entropy in a universe where entropy is, on the whole, steadily increasing. In their living processes organisms use the ebb and flow of entropy as it suits them.to maintain homeostasis in a changing environment. And of course the balance they maintain isn’t centred on a static mean, it progresses in a dynamic fashion.
Are you being deliberately vague here? Work is done by what on what? And how do you define the system? It makes not difference to an organism if an insignificant fraction of available energy in the universe is lost in order for it to have useable ATP molecules. Organisms are extremely adept at being able to convert and use energy in order to sustain their existence and living systems have advanced to the stage where energy has been harnessed to produce individual consciousness. It doesn’t matter to which side of the equations energy flow belongs, what matters is that consciousness is the result.
Because you have asked this question, I would guess that one produces energy and the other consumes energy. As far as assigning changes in entropy it would depend on what is included in the system being examined and what type of entropy is being considered.
That is why previously I linked to a paper which highlighted the confusion that ‘entropy’ brings:
They write regarding entropy:
There are so many ways of looking at entropy, configurational entropy, thermal entropy, Shannon entropy, residual entropy, total entropy, negentropy, electronic entropy, magnetic entropy, information entropy…
According to Sean Carroll there are basically four types of entropy, Boltzmann entropy, Gibbs entropy, Shannon entropy and von Neumann entropy. I would recommend anyone interested to watch that video. He discusses Maxwell’s demon, fridges, the von Neumann quote and much else besides.
It’s no wonder arguments about entropy become hard to follow for many onlookers, I’m sure.
Friedrich-Wilhelm Dustmann
The concept of entropy is fairly straightforward when dealing with such things as steam engines. But when it comes to living systems things get extremely complicated.
Cracking the egg and poking it involve mechanical work. Mechanical work produces heat. Perhaps the heat energy produced would be enough to break one or two chemical bonds locally. We all know the effects of boiling an egg I’m sure.
No, it doesn’t get that complicated. It’s a matter of having your chemistry/physics foundations right, as well as understanding that the analyses can be done at several levels, some far too easy to perform and understand. However, I’m just having fun with Nonlin’s petulant ignorance, so no further discussion from me to you.
That would be true if the computer were ONLY composed of screws. Which is what you insist the mind is: “just molecules”. Back to you.
So you think ‘A therefore B’ is SAME as ‘not A therefore not B’? Haha. Pre-K has a new old student. He will NOT be missed by his would be K teacher.
Cease and desist. “Haha” is trademarked.
Forgetful? This was not about spontaneous. It was about cooling a system in equilibrium at room temperature WITHOUT energy. Which you can’t. Of course, “having created” (God?) … Only that’s in another dimension.
Which you FALSELY claimed you could disprove. When will you? Never! And stick to the original scenario.
Exactly. Prove your claim “that FOR SURE no chemical bonds are broken when the egg breaks”. Are you retracting?
I can’t find and you can’t prove. When will you cut the crap?
Not this particular study. The main idea HERE is that entropy change is asymmetrical. And that critical evidence expected if abiogenesis were true, is in fact missing.
Forgetful again? The discussion was about supersaturation, not just any old crystallization. And indeed, there is an energy barrier there. So, as long as I have your agreement, back to abiogenesis and entropy IRREVERSABILITY. Remember, you also agreed abiogenesis is NOT crystallization.
Sean Carroll is a clueless poet of bad philosophy masquerading as a physicist.
Not according to Allan Miller. He will PROVE any minute now (or never) that not ONE single chemical bond is broken. Haha.
Entropy in physics is more than the cargo cult practice of taking the name of that which you don’t understand. Haha.
Boringly predictable. I knew you’d go for “ONLY”, after failing with that amazing change from “true” for “not true”, after failing with that claim that fallacies of composition did not happen until emergence was proven. But let’s check your logic again:
Wow, surely no longer a fallacy of composition!
🤣
I’ve never said such a thing. Since it’s the second time I tell you, I wonder if such a small sentence is too much for your capacity to read. Not that I’d be surprised.
So you think ‘A therefore B’ is a fallacy of composition? Your pre-K teacher must be sorry to have you back for the millionth time.
Do you ever get embarrassed when you screw up?
🤣😂😂😂🤣🤣
Physics foundations aren’t enough to solve the three body problem. Calculating energy levels in mechanical systems in relation to calculating energy levels in living systems would be comparable to working out two body interactions in relation to working out three body interactions.
Ilya Prigogine had some interesting ideas in this area. Mechanical systems are deterministic and so working out cause and effect is fairly straightforward, whereas living systems are much more probabilistic and so causal relationships are far less certain.
I’m sure Nonlin would be interested in this paper by Andy McKintosh on entropy in which he discusses Prigogine. That paper discusses two other ‘entropies’, logical entropy and Prigogine entropy.
Does that mean you don’t have fun with me and my ignorance? 🙁
Stephen Hawking wrote in his book ‘A Brief History of Time’ p108:
But what did he know?
And of course here we have another specific entropy, Bekenstein-Hawking entropy. From its inception by Clausius as simply a term for energy dissipation it has constantly grown ‘arms and legs’ to become the complex concept we grapple with today.
Simply not true.
https://en.wikipedia.org/wiki/Stability_of_the_Solar_System
You may imagine that the movements of the planets can be predicted to a high degree of accuracy over long periods of time. Simply not the case.
Chaos disproves your claims that mechanical systems are deterministic. Any orrery you create that is actually accurate will be unpredictable over the long term.
Let me rephrase my words, classical Newtonian mechanics is deterministic. I have already mentioned the three body problem regarding the motion of planets.
Your case is a bit more complicated. You believe some woo and make everything fit into the woo, even if it doesn’t fit. But the issue is: woo first, reality we’ll see. I doubt anything can be done about it, other than you realizing what you’re doing. Maybe you realize but find nothing wrong with your approach.
Anyway, have a great time.
Entropy,
You write too much desperate nonsense. Btw, did your superiors leave you all alone here?
It starts really, really bad: “Functional information and entropy”… There is no information – entropy link, Shannon’s METAPHOR notwithstanding.
Aiming for the irony of the year award? You already won that one. Actually, you filled all of the nomination spots. No need to try further.
Entropy,
Yeah, right.
Anyway, this is a tad intriguing. So what is the mind composed of if NOT “just molecules” (meaning a composition of known particles)? Be specific, and explain how you came about that knowledge.
Exactly right. I think you won for “Do you ever get embarrassed when you screw up?” But you had so many it must have been a hard competition against yourself.
I think that when people talk about the mind, they refer to an activity. Thus not something I’d refer to as “being composed of”, just like I wouldn’t refer to running as having a composition.
By reflecting about it.
Seems you can’t grok that I am saying the same thing now that I said then. If I was agreeing with you and keiths then, why have you changed your mind?
Then:
Yet you appear to be asserting that there is such a law. There is something that literally precludes the improbable from taking place.
And now we know that I have said what I think. so thank you for that acknowledgement. Not only have I said it in other places, as you well know, I have also said it in this very thread. Take for example the following:
You were strangely silent about that comment, did you simply overlook it? Do you disagree that entropy is a measure, and if so, why didn’t you speak up?
Allen thinks entropy is a measure of energy being dispersed. I think entropy is a measure, but not a measure of how “spread out” energy happens to be. I think keiths agreed with me. So again, I have stated what I think.
What I’d like to know is, why you think something other than probabilities is involved, and what is that “something” that you are appealing to?
How you define heat?
I haven’t. You need to pay attention better.
This ‘appearance’ is the result of your not paying attention.
I found it so banal as to be unworthy of comment. Like most of your recent product. Again, if you were paying attention you wold know that I view entropy as a book-keeping convention, albeit as unreasonably successful one.
That was years ago. On this thread you stated that it’s impossible for anyone to roll four sevens in a row, and that 2LoT prooves that the Dawkins Weasel program could not posslbly have produced the results it did [sic]… so out of three actual statements on this thread, two were utter rubbish. I can only assume that you were trying for irony. This is more effective if you are correct more often than you are incorrect. See phoodoo or nonlin for examples.
You are ascribing to me a position that I do not hold. Try to pay attention. Seriously, you used to be quite good at this, but these days your game is really, really weak.
It’s the difference between the internal energy change and the amount of work done on a system. Most easily thought of as the kinetic energy of the molecules. Or, to put it in Flanders and Swann’s language, “Heat is work and work is heat“. 😉
OMG! So I wrote Allen and not Allan while linking to a post which made it clear who I was referring to. So crucify me!
So now we know what DNA_Jock is all about.
Oh goody! So we’re all in agreement!
I had better fit or we’re on the wrong track. Fit into woo as in the (w)orld (o)verarching (o)urs. that is 🙂 And we have only just begun to scratch the surface, probe the depths and fathom the heights of this ‘woo’.
We see through a glass darkly, just shadows on the wall. The reality we see is very limited. It is a very gradual process whereby the pieces of it are revealed. Every schoolchild is now aware of things that neither Newton nor Darwin had a clue about. They learn about DNA and spiral galaxies and quantum entanglement and quarks and relativity and solar winds and many other things that were all parts of a reality that was above the heads of these giants of science. And the schoolchildren of the future will be equally aware of a reality unknown to the intellectual giants of the present.
I do try. There are a few here who would say that I’m trying 🙂