[Thanks to Alan Fox for asking questions about YEC and Elizabeth Liddle for her generosity in hosting this discussion]
YEC part 1 gave some theological and philosophical context to the case for YEC, and part 2 will hopefully focus solely on empirical and scientific considerations. Part 2 challenges the mainstream view that the fossil record is hundreds of millions of years old.
If empirical considerations alone suggest the fossil record is not more than a several million years old, does it matter on balance that the data don’t exactly arrive at 6,000 years old? I think not. As far as I’m concerned, if the fossil record is not anywhere near as old as the mainstream claims, the creationists will have won the essentials of their case independent of whether the universe and Earth are billions of years old. Creationists can afford to lose the issue of the age of the Earth and universe, but Darwinists cannot survive the fossils record being only a few million years old. But as I demonstrated in YEC part 1, time isn’t the friend of evolution anyway, it is an enemy since nature tends to erode complexity, not construct it.
If the age of a skyscraper built with 1 billion year old rocks does not imply the skyscraper was built 1 billion years ago, the age of the fossil record can be formally separated from the age of the universe and age of the Earth and age of rocks. The time of death of someone can be determined forensically and the process doesn’t rely on the age of the Earth or universe or rocks around deceased to make a reasonable inference. The age fossil record is about establishing the time of death of the fossil not the rocks the fossil is buried in. The age of when a strata was formed is independent of the age of the rocks that form the strata.
When I ask geologists how do permineralized or well-preserved fossils form. As a matter of principle, does the entombment happen quickly or slowly? “Quickly” is the usual reply. Why? Rapid burial with minerals and water are the necessary ingredients to effect preservation. If the creature dies and is left out in the open to scavangers and decay processes, it will not fossilize. So as a matter of principle, such fossil bearing formations didn’t take millions of years to form. Thus one can’t argue the fossil record is old because it took millions of years to bury them! In the case of wooly mammoths with undigested tropical vegetation in their stomachs, they’d have to be instantly buried in snow to effect the necessary freezing to preserve the vegetables in their stomachs — not millions of years. That’s the other thing, why are the mammoths in a tropical environment one moment and then buried in a cataclysmic blizzard the next, and then never unfrozen till discovered in Siberia? Hmm…..
So like a detective, we’ve established certain fossils are buried rapidly, not over millions of years. The question remains when it happened, or maybe when it couldn’t have happened.
The mean sea level of the US is here is around 760 meters. Erosion of a mere 10 microns per year would wipe 760 meters into the sea in 76 million years. A sheet of paper, by the way is 100 microns thick. The slowest mean erosion rate I’ve found in literature is 2.5 microns per year, and even that would wipe out the Phanerozoic in many areas.
I point to this empirical study by Princeton geologists Judson and Ritter: Judson and Ritter
Taking the average height of the United States above sea level as 2300 feet and assuming that the rates of erosion reported here are representative, we find that it would take 11 to 12 million years to move to the oceans a volume equivalent to that of the United States lying above sea level. At this rate there has been enough time since the Cretaceous to destroy such a land mass six times. Accepting this figure creates the problem of maintaining a continental mass above high elevations. A problem beyond the intent of this report
Granted, that may only be a mean value for now, but one can’t fight gravity, sediments will tend to move toward the oceans, erasing the fossil record in the process. Even if Judson and Ritter are off by a factor of 50, that would still wipe out the fossil record all the way to the beginning of the Cambrian.
But even more to the point, we have forensic clocks that may put an upper limit to the time of death of the fossil in question. There is the very embarrassing fact that the supposed carboniferous era of 300 million years ago has ubiquitious traces of C14, and this is acknowledged in peer-reviewed literature. 0.1% present day concentration of C14 will yield a presumed age of 57,000 years. That is 1 part in 1000. We have frequent detections of comparable levels, so much so many won’t even try to date with C14 beyond that presumed age because there seems to be a persistent amount in fossils!
Some claim contamination, but this explanation is not as credible as one might suppose.
First consider in-site contamination. To maintain a background persistent concentration of C14, one needs to keep adding more carbon from atmospheric sources into the fossil to maintain 0.1% concentraion. The problem with this scenario however is that the added C14 will decay away, and one needs to add even more carbon contaminants the next iteration to maintain a background C14 level of 0.1%. One ends up with something analogous to the compounding interest rate problem. Say I added a mere 0.1% contaminant every 50,000 years, over 300,000,000 years, the fossil will either gain 402 times in mass or be diluted from the original material by that factor. Maybe in-site contamination might work as an explanation for isolated cases, but not for repeated discoveries in diverse geographical locations, otherwise one would have to argue nature conspired to fool us by contaminating the entire world recently for no good reason.
Consider contamination during processing of the fossil. 1 part in 1000 might seem like very little, but consider contaminating a hard piece of fossil marble or shell or bone. Just to illustrate, take a 1 gallon (not quite 4 liters) sample of something hard. A little less than a small teaspoon (4 milliliters) of contaminant to 1 gallon would be 1 part in 1000. Do you think you can force that much contaminant into something relatively hard? 🙂 Even 1 teaspoon into 10 gallons wouldn’t exactly be easy (1 part in 10,000). So this is not as credible an explanation as would be supposed either. Are experiments and analysis actually done to determine the source of contamination? No, because the fossils C14 is primarily due to contamination, it is due to the fact the fossils are young. And few are willing to stick there neck out to point out they can’t demonstrate the source of supposed contamination.
Radioactive decay chains have be also ruled out unless of course one assumes 99% Uranium and less than 1% of fossil!
See:
Problems using Coal as a C14 free source
Lowe points out:
Many (super 14) C dating laboratories have established that coal samples exhibit a finite (super 14) C age, apparently caused by contamination of the specimens before any laboratory preparation is undertaken.
He then points out the contamination cannot be due to radioactive decay of other products:
Because coal is formed over geological time scales at depths providing excellent shielding from cosmic rays, its 14C content should be insignificant in comparison to the 14C introduced by even the most careful sample preparation techniques used in 14C dating laboratories. How is it then, that a material, which should show a14C age indistinguishable from that produced by a combination of machine background and contamination during careful sample preparation, routinely produces a finite 14C age?
One suggestion is that radium, which is present in some coals at the sub pm level, as a decay product of the uranium/thorium series, may produce 4C during an extremely rare decay event (Rose & Jones, 1984). Jull,Barker and Donahue (1987) have detected 14C from this process in uranium/ thorium ores. Blendowski, Fliessbach and Walliser (1987) however, have shown that the 14( decay mode of 226Ra is only of the order of 10-11 of the preferred a decay channel to 222Rn. Thus, the amount of 14C produced by such events derived from radium in coal must be considered as insignificant.
and finally capitulation at the ubiquity of the problem
There are many other unpublished accounts by 14C laboratories in which the use of coal as a background test material has been investigated. In many cases, the samples were found to contain 14C, and further studies were discontinued. The AMS and gas counting facilities, DSIR, in Lower Hutt, New Zealand, eg, have observed apparent ages for coal specimens ranging from 25-40 kyr, and the NSF Accelerator Facility at Tucson, Arizona has determined ages of anthracite samples ranging from 30-40 kyr (AJT Jull, pers commun, 1988).
Lowe invokes bacterial contamination, but I pointed out why such in site contamination is contradicted by the “compounding interest” problem, not to mention, bacteria in deep parts of the Earth would be feasting off C14 depleted carbon, not atmospheric carbon!
Next is the fact of biological materials with half-lives that preclude their persistence in fossils. DNA has a half life 521 years give or take, homochiral amino acids have half-lives on the order of hundreds or a few thousand years. The state of these biological materials in fossils is inconsistent with the time of death hundreds of millions of years ago.
Additionally, we have ancient fossil DNA that looks like modern DNA, breaking the biological molecular clock hypothesis. See: Pardox of Ancient Bacterium. But detractors bring up the contamination complaint yet again.
The actual forensic clocks refute old age fossils in the fossils themselves (C14, DNA, homochiral amino acids, inconsistency with biological molecular clock). The well preserved variety of fossils could not have been buried in a process taking millions of years as a matter of principle, and there should be serious doubt the fossil record would still be around after hundred million years, maybe not even 11 million years.
Finally, I keep hearing assertions about all the mounds of data that prove the fossils are old. Actually it’s mounds of publication not mounds of actual facts. The amount of words dumped out does not necessarily make claims any more credible than Kairos Focus being verbose proves Kairos Focus’ FYI/FTR are correct. It’s the physical facts that count. The tons of fossilized material themselves do not indicate an age that is as old as most presume.

Since I have pointed out why this is false, with several references, I can only conclude that you are deliberately…
Nope, Lizzie would get mad. But that’s what you are doing.
When you get around to the last 50 or so years of radiometric dating, and address the consilience between K-Ar dates and the many many more results of more robust methods (radiometric and non-radiometric) we could have a discussion.
But you can’t discuss, all you can do is regurgitate.
Don’t bother, I (and I suspect many others here) understand the obsolete K-Ar method well, as well as the ridiculous objections other YECs have raised and which have been debunked decades ago.
Address the U-PB concordia-discordia method first. Then the Ar-Ar method. There are many others, but those are the big two. Not K-Ar.
Lord have mercy on our souls, the helium in the atmosphere PRATT! From ol’ Melvin himself! You really have no shame.
From Accumulation of Helium in the atmosphere. Dalrymple’s full discussion can be found at EFFLUX OF 4He INTO THE ATMOSPHERE.
Actually, Cook’s claims were published in Nature in 1957.
He was wrong.
This has been demonstrated.
But YECs don’t care.
The lies are lies of omission, from AIG and and someone more directly connected to this thread.
But maybe not buried rapidly. Got any evidence? Of course not, your loony sources don’t,and you haven’t done any thinking or research into it.
There was one. Millions of years ago.
Sal seems to have gone into a mode where he ignores what everyone else is posting (and in particular he ignores questions). Instead, he is posting a series of “sermons” which seem to be taken from ICR and AiG.
Entertaining, perhaps. But not informative.
Depends. In many cases from groundwater. Some bones have been dated by U-Th disequilibrium dating,. Groundwater containing soluble uranium perfuses tthe bone, uranium decays to insoluble thorium which precipitates into the bone, and is therefore not in secular equilibrium. the bone can be dated by measuring how far it is from secular equilibrium. In fact, there is a depth profile in the bone that contains even more information. E.g. Absolute dating by uranium series disequilibrium of bones from the cave of La Chaise-de-Vouthon (Charente), France. The method can be used for ages well beyond the range of 14C.
Not that Sal would understand any of that.
But the point really is that the simplistic assumption that the only source of carbon in bones is the bone itself is not justified.
Prove it.
They also found miraculously preserved proteins.
Dinosaur bones in marine environments?
Reminds me of marine microfossils in tree resins.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2582268/
They say high tides got into the resin flows that made ambers.Anyone been on the beach seeing ambers form in high tide?
Ambers are not easily formed.
We don’t have marine microfossils accumulating for millions of years on the sea floor, we have them being involved in the rapid burial of land-based plants and animals.
Heard from creationists friends, marine fossils almost always mixed in with those rapidly buried land fossils.Recall the OP said well-preserved fossils must be rapidly buried. Apparently they are buried not by landslides but by big movements of water that can put marine fossils in the same vicinity.
There does not appear to be a slow gradual formation of the fossil record, but rapid cataclysmic death.
I know the rules say that we are to assume that other participants are commenting in good faith, but I’m almost ready to call Poe. Everything that Sal is throwing up here was addressed decades ago on the talk.origins Usenet newsgroup. It’s so thoroughly debunked that the talk.origins archive has the responses summarized in the Index of Creationist Claims.
There is no reason to waste time on this nonsense (although I appreciate some of the more detailed refutations being posted). Just point to the archive and laugh.
I will merely point out that Sal persists in posting screeds by people who quote mine, who engage in academic fraud (actually changing the words attributed to scientists), and posting arguments that have been addressed decades ago.
Without dealing with already published responses. Since I am forbidden from attributing this to dishonesty, I attribute it to discourtesy.
JonF,
You have not proven K-Ar dating is accurate, in fact you have provided plenty of reasons to show it is flawed through contamination. Even after you listed some possible mechanism of contamination as corrupting K-Ar, you insist it is accurate and the dates aren’t cherry picked. Don’t you see the irony.
The problem is Argon 40 is stable, whereas C14, amino acids and DNAs are not, so even if contamination happened, one has to account for why the contaminant is still young when there is no mechanism for sustained renewal of the contaminant.
Being mistaken is not the same as being dishonest. If I believed in my heart you guys were right and only pretended I didn’t agree with you that would be dishonest.
I don’t believe you guys are right. I’m continuing to state the reasons why.
If you don’t like it, you can of course not participate in this thread. I’m going out of my way to compartmentalize some of the detailed YEC discussions to a few threads like this one that is already off the front page, so that you can view another channel if you don’t like my monologues.
What I see however, is you guys aren’t having as easy a time dispensing the issues as you’d like. If you really had a case, you wouldn’t have to put up the dishonesty card, you’d demonstrate it with hard science, which so far hasn’t been conclusive for the Old Fossil Record case.
I think I’ve shown, the fossil time of death, remains uncertain. In the USA, the death sentence can be avoided if reasonable doubt is introduced. I think I’ve shown there is reasonable doubt as to the time of death of the fossils, and the Young Fossil Record claim should not yet be sentenced to lethal injection.
Yes, I have. Consiience. Address consilience. None of your cherry-picked claims trump consilience
No. I acknowledge that is is possible that some K-Ar dates are wrong because it is not as robust as the many other methods, and we know of some K-Ar dates that are wrong and (for most of them) we know why. It’s not because the Earth is young.. I acknowledge that several YECs have found anomalies (very very few, once you remove the outright frauds and intentional misrepresentations {such as your citation of Hawaiian lava from 1801}). However, given the fact of consilience with other more robust dating methods and non-radiometric methods, and Dalrymple’s investigation of recent lavas and the lack of significant excess argon in almost all of them (which I’m surprised you haven’t yet mischaracterized as so many YECs have), it is not physically possible that even a large proportion of K-Ar dates are wrong.
You can’t address the important facts; the many more widely used and more robust methods, and the consilience between methods that (among other things) proves that K-Ar is generally reliable and the Earth and life are old. Most likely because almost all of the YEC sites from which you are blindly cutting and pasting also don’t dare address the importatn facts. The only YECs who have the knowledge and the balls to address the facts was the RATE group: From Helium Diffusion Rates Support Accelerated Nuclear Decay:
They realized that only possible reconciliation of a young Earth and radiometric dating is Accelerated Nuclear Decay( AND). Boy oh boy, would I love to talk AND with you! Or anyone.
Your claim of cherry-picking is still unsupported and odious (but who expects anything else from you?). You have cast unwarranted aspersions on thousands of honest scientists just to avoid honest questioning of your silly preconceptions. Slimy Sal indeed.
I know Sal won’t even read this, but Dalrymple’s results are interesting. He surveyed 26 subaerial* lava flows using the Ar-Ar method which detects “excess” (initial) argon. 18 of those (70%) had no excess argon or so little excess argon that it would not affect a K-Ar date. Of the remaining eight, seven had some excess argon but not enough to affect a K-Ar date if the rock were a few millions of years old, and one had quite a lot. That was the Hualalai flow, known for xenoliths (does Sal know what xenoliths are yet?) and fluid inclusions containing argon. I can supply a copy of the paper if someone supplies an email.
————————-
*Subaqueous or “pillow” lava forms an outer shell immediately on contact with the water, trapping excess argon inside on a regular basis. Geochronologists know this, YECs don’t.
Consillience? How about peer-reviewed enforcement of a narrative where conflicting data are edited out and explained away. You’ve given lots of reasons to distrust K-Ar dates, and you don’t see the irony!
You complain of contamination of C14, amino acids, DNAs which cannot possibly be sustained because even if the contaminants were there, they have half life, and yet you go out of your way to say there is no Ar40 contamination and yet give lists of references showing there is credible reason to invoke Ar40 contamination (which cannot decay away like C14, amino acids, and DNAs) and how cherry picking can easily happen. You’re the one running from reality.
What’s comical is how quick you invoke the contamination argument for the 3 clocks I put forward for fossils, and how quickly you run away from credible empirical and theoretical evidence of Ar40 contamination! The problem is A40 contaminants don’t have a half lives like the clocks I’m invoking. Too funny.
Only if you ignore the discussion and references provided to date. Do you still claim that K-Ar is a widely used dating method? Yes, you do. Is it? No, and I’ve proven that with discussion and references. How do you explain your continued presentation of a known falsehood? (I predict Sal will not attempt an explanation.)
We don’t need the ‘dishonesty card”, we just note it where YEC dishonesty is blindingly obvious. The fact that you are stunningly dishonest does not prove an old Earth and nobody is trying to use it as such. The evidence we have presented and you have ignored is sufficient to disprove your claims. Of course you are playing your You gys are grasping” card to avoid the facts.
Nobody else does. Debunked PRATTs from decades ago are not convincing, and that’s all you’ve presented. I suggest Introspection.
Science is not a court of law. “Reasonable doubt” does not obviate the hundreds of thousands of undoubted facts that you ignore and that prove that the Earth and life are old. Your doubts have been shown to be unreasonable.
No need to sentence a young fossil record to lethal injection, it died of natural causes nearly two centuries ago.
YEC is perhaps the greatest conspiracy theory of all.
Answer:
Actually that’s far more problematic for Old Fossil record interpretation because of the great Unconformity:
http://www.lpi.usra.edu/science/treiman/greatdesert/workshop/greatunconf/index.html
Where did the sediments come from to create global sedimentary layers that match across continents? Were there fortuitously the same sort of mountains containing the same sediments in geographically separated regions that provided sediments to fill in huge areas at the same time?
As I pointed out here
Slow steady tectonic movement is not indicated by things like the Permian basin. For that matter, the Great Unconformity. The hundreds of millions of years missing from the fossil record as evidenced by the Great Unconformity are because there were no hundreds of millions of years in the history of the fossil record.
The great Unconformity and places like the Permian Basin
http://theskepticalzone.com/wp/wp-content/uploads/2015/06/west-texas-structure-the-university-of-texas-of-the-permian-basin-1062×3441.jpg
Are consistent with the idea the sediments were laid down about the same time and then uplifted afterwards about the same time.
Uniformity happens quite easily through fast stratification as demonstrated in lab experiments at Colorado school of mines and observed by Walter Law.
https://en.wikipedia.org/wiki/Facies#Walther.27s_Law_of_Facies
This Youtube shows experiments and field observation confirming Walter’s Law:
Unsupported assertion. Please provide evidence that conflicting data are edited out without objective, stated, and valid reasons for it.
I’ve given plenty of reasons to trust K-Ar dates, and you have ignored them. You are terrified of addressing the facts of consilience and the dating methods that are actually used.
I have not shown any lists of references showing there is credible reason to invoke 40Ar contamination. I have provided lists of references which demonstrate that significant 40Ar contamination is extremely rare, and lack of 40Ar contamination is quite common. Exactly which of my lists and references ” there is credible reason to invoke Ar40 contamination”??
Yup, it cannot decay away. But in the much more widely used Ar-Ar method it can be detected and often a valid date can be obtained. 40Ar/39Ar Dating into the Historical Realm: Calibration Against Pliny the Younger:
(emphasis added, full text available with free registration)
IMHO and with my obvious far greater knowledge of the field I think it would be very difficult to cherry-pick results. You still haven’t presented any evidence that cherry-picking ever happens, much less to any significant amount. Remember that you need to disqualify all the radiometric dates, a few anomalies don’t cut the mustard.
Oh, Sal, you are such a card. You repeatedly post falsehoods after being proven wrong, you cut and paste long-debunked PRATTs without questioning or even understanding them, you gallop like a Gish, and you refuse to provide evidence or argument for your crazy and odious claims.
Yeah, I’m the one who’s running away by presenting evidence and discussion that refutes your claims. Sure, Sal, sure.
…
We have presented evidence for contamination in your other clocks.
Excess argon occurs. Nobody has denied that. But there is incontrovertible evidence that it’s moderately rare, and the majority of K-Ar dates are not affected.
You are still running like a scared widdle bunny-rabbit from the radiometric methods that are actually used and the consilience between them. Even if nobody had ever performed a K-Ar date, we know that the Earth and life are old.
Ar-Ar dating. U-Pb concordia-discordia. Consilience. Look Jane! See Sal run! Run, Sal , Run! Sal is running!
I don’t think there is any evidence that will make Sal change his mind. He’ll clutch to any doubt he can find. Dogma. All of science must be wrong.
Life would be boring without a few flat earthers to kick around. I have, however, run into geocentrists who could argue without quote mining.
But Sal can be educated. He figured out 2LOT. Geology should be next..
Like Glen Davidsons claim Si02 can increase C14? 🙂
Like your non-answer on requisite uranium concentration?
Like your solution to the “compounding interest” problem posed by clock materials with actual half lives?
You’ve done a good job showing how easy it is to mix some argon bearing material into a sample and creating a false date. Yet you’ll swear there is insufficient contamination because to look at the evidence upsets your doggedly held beliefs, even after you yourself gave mechanism showing how easily it can happen. Ar40 is almost 1% of the Earth’s atmosphere, and it doesn’t have a half-life like the C14, amino acid, and DNA clocks. You just ignore the significance of the fact Ar40 has no half life yet the clocks I reference for fossil time of death do.
And Uranium Lead dating of fossils? I’m not aware that Uranium is a significant component of living creatures? You’re dating the time of death by the Uranium Lead dates of the rocks a creature is buried in rather than using the actual clocks that are embedded in the fossils themselves? Not wise.
I think willful ignorance is dishonesty.
So if I understand the argument correctly, the scientific literature which establishes an old earth cannot be trusted because it excludes contrary results, and we know this because we can quote-mine that same literature for contrary results. Catch-22 or incoherent self-contradiction. Either way YEC wins.
So how did Sal come to understand 2LOT? Maybe take the same approach here that was taken then. Or is that what folks ARE trying to do? But for some reason I think Sal was probably more open-minded about 2LOT.
Sal combs the published literature for examples of evidence for YEC that have been suppressed by the cabal. Finds no shortage of discrepant data that has been purged from the documents he cites.
Newton, by the way, probably cleaned up some of his observational data. Proof of geocenttism.
Moved a post by Glen to guano. Glen, you’ll have to make your point without accusing a fellow poster of “flat out lying”, though I fully appreciate the difficulty.
So Sal can just lie about someone else, and can’t be called out on it?
So libel’s fine, complaining about libel’s not fine?
Lizzie quit pretending that Murray is commenting in good faith, but even that wasn’t for just making a total fabrication. Oh hey, not guanoed. Sal makes a complete lie about me, I call him on it, oh, it’s got to be guanoed. Screw consistency, and be sure to let liars lie, I guess.
What the hell is someone supposed to do here when lies are being told in hatred and bad faith by one of the slimiest a-holes in existence? So libel is just fine with TSZ, just don’t call lying slime on their out-and-out smears.
So send this to guano, and face up to the fact that lying about another commenter is allowed in regular threads, and the truth about someone else is only allowed at guano. Sort of the honestly level of lying Sal.
But why can’t you put in the rules that flat-out lies about other commenters are allowed, while any defense is not?
Glen Davidson
Oh, how is flat-out lying supposed to be labeled?
There really isn’t any way of calling what Sal did anything less than sleazy dishonesty, and like terms.
Glen Davidson
GlenDavidson,
As I said, Glen, I fully appreciate the difficulty. Raise it with Lizzie in the moderation issues thread. There’s no problem with setting out the misrepresentations and distortions as forthrightly as you like. The rule is that nobody should accuse a fellow commenter of posting in bad faith. I’m sure fair readers will join the dots for themselves.
No, libel is not fine. If you have been misrepresented in these columns you are entitled to insist on a retraction and correction.
The thing is, he made the same reprehensible claim while quoting me in the past. All I had to do was to point to the rest of the sentence.
Now he just states it without the quote, apparently because the quote showed how wrong his claim is. I suppose I could dig up quotes and what-not, but honestly, why? It’s not like he’s trying to make an argument or a point, he just wants to smear. How am I supposed to treat it as if it’s in good faith when it’s clearly anything but good faith?
It’s the old “have you stopped beating your wife?” thing.* Attack falsely, let the other person flounder around looking pathetic, arguing about what was said, and when. If that’s what’s going to happen here, how is anything actually going to occur in good faith? We’re supposed to pretend by responding to a “have you stopped beating your wife?” sort of charge?
Basically, it was the same with hotshoe, keiths, and mur2 as well when arguing pronouns for God. Nothing I wrote was ever taken in good faith, and was twisted into what it was not. Lizzie twisted again and again as well, but I think that was more carelessness than deliberate, although hardly taking what I wrote in good faith, while keiths, hotshow, and mur2 were simply reprehensible.
Really, there has to be some limit to bald-faced lies. It’s unfair to expect the one lied about to go on explaining as if the lie was told in good faith.
Glen Davidson
*Well, not exactly the same, but pretty close, since responding to a fabrication gives credence to that fabrication.
GlenDavidson,
I won’t address the off-topic issue you raise here. As to Sal misrepresenting you by as you put it;
You are referring to this;
So let’s see if Sal can do the decent thing and apologise for misrepresenting your views. Sal?
Well then, since the following is completely untrue, retract and correct this, Sal:
So what do I do if he doesn’t?
Glen Davidson
GlenDavidson,
I think if Sal doesn’t respond, he is acting in bad faith. The misrepresentation and any repetition would be guano. I hope Lizzie will rule on the rule here.
Lets raise it in the moderation thread. I will support you.
petrushka,
OK
Scripture states that no liar will enter the kingdom of heaven. So while asking for a retraction might be all nice and help set the record straight, what is really needed is repentance, which would entail a change of heart. That way both parties benefit.
But if all you want is an retraction that’s all you’ll be likely to get.
.02c
Just got back from les Alpes (where I wandered over fecking MOUNTAINS of neatly stratified fossils made of magically fixed carbon and calcium), and I see I have a full week’s worth of defending the indefensible to get through.
First, coffee.
I cannot grasp the mindset of someone who posts 30 year old quote mines from the dumbest fucking creationist websites, and at the same time taunts fellow creationists about 2LOT.
stcordova,
I think the problem is that ‘hard science’ simply bounces off your YEC armour. You have a hopelessly naive view of sedimentary processes, of contamination issues, of the relative merits of racemisation over various isotopic methods, of fossilisation (you really can’t see how flying animals can end up at the bottom of a lake when dead … ? 🙂 ) … you’re a YEC, and a YEC you will always remain, for reasons best known to yourself. The problems you articulate are not real problems. They are problems arising from a distorted view of the various sciences on which you cast your gimlet eye, a view no-one but yourself can correct, and you’re the last person who seems to want to try.
Ar-Ar. U-Pb concordia-discordia. Consilience. See Sal Run!
I did not answer your question on realistic uranium concentration. I pointed out that it was irrelevant because it addressed a different scenario than I proposed. Your non-answering repetition of the same irrelevant question is the issue there.
Ar-Ar. U-Pb concordia-discordia. Consilience.See Sal Run!
Not a problem. Based on a false assumption of a possibility adding significant contamination after solidification.
Ar-Ar. U-Pb concordia-discordia. Consilience. See Sal Run!
No. Nothing you or I have posted indicates that is even possible to add significant argon to a rock after it solidifies. Everything I have posted relates to “excess” argon present at solidification.
If you wish to argue that significant argon is added after solidification, show us the mechanism, show us the calculations, show us evidence that it actually happens, and show us evidence that it affects Ar-Ar dating. (Especially step-heating plots.)
You can’t. All you can do is cut and paste creo crap. And no other YEC has done this for you.
Ar-Ar. U-Pb concordia-discordia. Consilience. See Sal Run!
We know ther isn’t significant contamination. Consilience between many radiometric and non-radiometric methods. How does 40Ar affect all those methods equally?
You haven’t presented any evidence that significant argon can be added after solidification. Nor have you addressed Ar-Ar, U-Pb concordia-discordia, and consilence (which is only a fraction of the available evidence.)
It’s called “projection “. Just because you refuse to even acknowledge the existence of the evidence, much less examine it, doesn’t mean that others do.
Ar-Ar. U-Pb concordia-discordia. Consilience. See Sal Run!
Nope, I gave no such mechanism. I didn’t even discuss the idiotic idea that argon can be added in any noticeable quantity after solidification.
Of course, if that did happen it would show up on step-heating Ar-Ar plots. But you have no idea what those are.
Ar-Ar. U-Pb concordia-discordia. Consilience.See Sal Run!
Tain’t significant. If you want to claim it is, let’s see mechanism, calculations, evidence that it happens and explanation why it doesn’t show up on step-heating Ar-Ar plots. Oh, and why if it did show up on those plots it would necessarily interfere with producing a valid date.
Obviously you aren’t aware that geochronologists know how much of each argon isotope there is in the air, and the requisite small correction for atmospheric argon contamination is so routine it’s almost never mentioned in papers, just textbooks and instruction for carrying out the test. But the correction is always small, never approaching the many orders of magnitude you need.
Ar-Ar. U-Pb concordia-discordia. Consilience. See Sal Run!
Not much uranium in living creatures. As I already pointed out, bones have been dated by U-Th disequilibrium dating due to uranium in groundwater decaying to thorium which precipitates out. I even gave a reference.
Do try to keep up.
Ar-Ar. U-Pb concordia-discordia. Consilience. See Sal Run!
As I pointed and you ignore, some fossil dates are directly obtained from U-Th disequilibrium dating.
You still haven’t given any reason for anyone to believe that geologists can’t tell the difference between a fossil buried before solidification, a reworked fossil, and a fossil buried by some disaster log after it died. Hint: they can, and your ignorance does not trump reality. Not wise.
Ar-Ar. U-Pb concordia-discordia. Consilience. See Sal Run!
Some of the scientists here may be interested in how K-Ar dating is actually done. Sal excepted, of course.
Radiogenic Isotope Geology is the textbook. There’s a version online, missing just the final tweaks of the 2005 edition and with some lousy formatting of equations. Of course he covers K-Ar, but as I’ve demonstrated it’s mostly for historical reasons. From section 1.1 (without the figures and the equations are toast):
From section 10.2 on Ar-Ar:
Run, Sal, Run!
I really appreciate the efforts of people to respond to Sal. He could have looked this stuff up himself, but he chose not to.
On a recent holiday, while gazing out from one of the many limestone escarpments in the Alps, the following calculation struck me, though I had to get home to plug in some values.
Estimate of carbon content of current biomass: 560 billion tonnes x 3 = 1,680 billion tonnes. (Source, Professor Wikipedia – 560 billion tonnes excluding prokaryotes; prokaryotes estimated to equal or exceed that, so let’s say double to be generous).
Carbon’s contribution to total mass of calcium carbonate: 1/6th
So 1680 billion tonnes of carbon entirely locked in carbonate would give 1680*6 =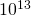 tonnes of limestone, assuming no non-carbonate contaminant.
tonnes of limestone, assuming no non-carbonate contaminant.
Kg of solid limestone per cubic metre: 2611, ie 2.611 tonnes
So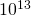 tonnes is
tonnes is  cubic metres.
cubic metres.
If this were a single square block 1000m high it would be just 62 km on a side. You don’t have to travel far through the Alps to see that kind of mass, and there are many, many more limestone regions globally to account for.
Of course I’ve made some ridiculous simplifying assumptions, some of which would inflate the volume – I have assumed ‘pure’ calcite, for example, and ignored non-biogenic limestone which is a substantial fraction of the total ( … though just how do you get non-biogenic limestone in a Creationist/Flood model, YEC-ers?).
But these factors are far outweighed by assumptions going the other way – that the entire carbon content of living things was devoted to making shell in marine environments, that every shell was fossilised, and that all the limestone there ever was is in a ‘quarriable’ form at the surface, none having ever been eroded or buried (you only have to stand on the edge of an escarpment, follow the line of a dip below other sediments, or go caving, to know what a rubbish assumption that is).
Be all that as it may, maximally efficient conversion of living carbon into calcite could barely account for the superficial geology of a country as tiny as little old UK. Where did the rest of the world get its carbonate from?
Of course I haven’t even mentioned calcium/magnesium or CO2 fluxes, or the requirement for shallow warm deposition conditions – no Gish-galloper I! But on sheer volume alone, carbonate rock appears to blow YEC out of the water.
I mentioned the “compounding interest” problem with respect to C14.
I use the phrase “compounding interest” since the problem is analogous to the growth of an account via compounding interest. If we invest $1 with a 10% compounding interest, after 100 years the value of the account is:
$1 x (1.1)^100 = 13780.61
To understand the problem physically, consider trying to keep a collection of water in a container cool by adding ice. When you add ice to it, when the ice melts, you have more water. Thus to keep the collection of water cool you have to add even more ice than you did the previous time. Each time you repeat the cycle you have to add more and more ice to have the same temperature effect.
Since C14 decays out of carbon, the carbon remaining is C14 free and one needs to add even more modern carbon to increase the C14 concentration to some trace amount (like 1 out of 1000).
All the stuff you cut and pasted invokes this circular reasoning and you don’t even see it. The sample could be young, and therefore the attempted correction is invalid.
I’m still here, and I pointed out again errors of circular reasoning, not to mention, even you accept how easily K-Ar dating can be contaminated. The issue gain is K-Ar dating involves daughter products that can be contaminated, and the contamination is persistent and additive since AR40 is stable, whereas C14, amino acid, and DNA have half-life decays and contamination fails as an explanation because of the compounding interest problem described in the previous comment.
Rocks recently out of the lava/magma phase will have very little argon to work with, thus it is sensitive to contamination since it is difficult to apply a correction. So the way then dates are accepted is to cherry pick the things that agree and through out those that don’t and give the impression that there are only a few outliers.
The K-AR correction assumes the contamination is atmospheric, as in the case of the Hawaii samples, the Older rocks might have provided a source of contamination that won’t be corrected by the AR36 method. Again, that’s the problem with the sort of contamination by stable isotopes or chemicals vs. decaying isotopes (C14) or chemiclas (amino acids and DNAs).
And again, the age of a rock above a fossil doesn’t take priority of the age the fossil itself. A living dog can be buried under a 65 million year old rock. Big deal! The age of the rock it is buried under. Doesn’t imply the dog is 65 million years old. The lack of logic is breath taking.
No, you are just misinterpreting what I said. There could be rocks that are dated older than the strata below them, in fact there could legitimately be an older rock on top of a younger strata or fossil.
The time of death of the fossil establishes the age of the strata.
There are known situations where a supposedly older layer is above a younger one.
http://creationwiki.org/Geological_column
Goes to show there are mechanisms which can put something dated older above something dated younger. The true age of the time of death of the fossil is the fossil clock itself, why go through the insanity of dating rocks when you can directly date the fossils themselves?
Answer: Fossil are not dated directly when they can be, that is exactly the problem.
I just gave a few examples with overthrusts, and who knows what other mechanism where even by mainstream standards old age rocks lie above young strata.
So why date rocks to establish the time of death of the fossil when there are clocks in the fossils themselves that establish the time of death?
Moved a couple of posts to Guano. Remember the rules, guys.
Sal, first of all, describing control of contamination as “cherry-picking” is grossly unfair. You imply that scientists decide in advance the answer they want, and then throw out the samples that don’t fit (William alleged something similar in the AGW thread). That is NOT how outliers are dealt with in science.
As an example in a field more familiar to you: let’s say someone tossed a coin 500 times (:)) and got 499 heads. The evidence is overwhelming that the coin has two heads – but one of the tosses produce tails. Do we throw out the two-headed hypothesis? Or do we investigate the event that produced “tails” to see whether the sample might have been contaminated by a regular coin?
“Outliers” are not merely “inconvenient data points that spoil our theory”. The proper definition of an outlier is a datapoint in which it appears that something else is going on – a piece of lava that includes a different type of rock, for instance; or a dog that got trapped in a rabbit hole (or a rabbit! There’s your “rabbit in the pre-cambrian”!) So what you do, usually, is investigate the outliers. Or, alternatively, you find yourself with a model in which a linear fit is excellent to 99% of the sample but not with 1%, so you simply posit two processes: a linear process and some other process. One candidate for that other process might be contamination. But it would be bad science to throw out the linear process which clearly fits most of the data just because some other process is clearly involved in a minority of it.
The second issue, which is what really does for YEC, is, of course, consilience. Why should we get the same answers for lake varves, coral layers, tree-trings, ice-cores, all of which indicate the same process going on for far longer than can possibly fit a YEC model? And that’s only for relatively recent records (tens of thousands of years, extending to hundreds of thousands in some lakes), but which corroborate different dating methods. Then there’s the tectonic plate evidence, which agrees, and cosmological evidence. Why should so many independent lines of enquiry give corroborating answers, if the answers were artifacts of methodology?
YECs always point to “outliers” – odd datapoints that seem to falsify an Old Earth, ignoring this vast consilience. When independent data paint one clear, coherent picture, then the way you investigate “outliers” is to find out why those do not fit the picture. And usually the answer is quite simple, whether it’s outlying radiometric dates, or polystrate fossils. It is far more parsimonious to conclude that one of the coins had one head, than to throw out the consilient evidence that the the vast majority of them had two.