During the past few days, Dr. Brian Miller (whose Ph.D. is in physics) has written a series of articles on the origin of life over at Evolution News and Views:
Thermodynamics of the Origin of Life (June 19, 2017)
The Origin of Life, Self-Organization, and Information (June 20, 2017)
Free Energy and the Origin of Life: Natural Engines to the Rescue (June 22, 2017)
Origin of Life and Information — Some Common Myths (June 26, 2017)
Dr. Miller’s aim is to convince his readers that intelligent agency was required to coordinate the steps leading to the origin of life. I think his conclusion may very well be correct, but that doesn’t make his arguments correct. In this post, I plan to subject Dr. Miller’s arguments to scientific scrutiny, in order to determine whether Dr. Miller has made a strong case for Intelligent Design.
While I commend Dr. Miller for his indefatigability, I find it disappointing that his articles recycle several Intelligent Design canards which have been refuted on previous occasions. Dr. Miller also seems to be unaware of recently published online articles which address some of his concerns.
1. Thermodynamics of the Origin of Life

White smokers emitting liquid carbon dioxide at the Champagne vent, Northwest Eifuku volcano, Marianas Trench Marine National Monument. Image courtesy of US NOAA and Wikipedia.
In his first article, Thermodynamics of the Origin of Life, Dr. Miller critiques the popular scientific view that life originated in a “system strongly driven away from equilibrium” (or more technically, a non-equilibrium dissipative system), such as “a pond subjected to intense sunlight or the bottom of the ocean near a hydrothermal vent flooding its surroundings with superheated water and high-energy chemicals.” Dr. Miller argues that this view is fatally flawed, on three counts:
First, no system could be maintained far from equilibrium for more than a limited amount of time. The sun is only out during the day, and superheated water at the bottom of the ocean would eventually migrate away from any hydrothermal vents. Any progress made toward forming a cell would be lost as the system reverted toward equilibrium (lower free energy) and thus away from any state approaching life. Second, the input of raw solar, thermal, or other forms of energy actually increase the entropy of the system, thus moving it in the wrong direction. For instance, the ultraviolet light from the sun or heat from hydrothermal vents would less easily form the complex chemical structures needed for life than break them apart. Finally, in non-equilibrium systems the differences in temperature, concentrations, and other variables act as thermodynamic forces which drive heat transfer, diffusion, and other thermodynamic flows. These flows create microscopic sources of entropy production, again moving the system away from any reduced-entropy state associated with life. In short, the processes occurring in non-equilibrium systems, as in their near-equilibrium counterparts, generally do the opposite of what is actually needed.
Unfortunately for Dr. Miller, none of the foregoing objections is particularly powerful, and most of them are out-of-date. I would also like to note for the record that while the hypothesis that life originated near a hydrothermal vent remains popular among origin-of-life theorists, the notion that this vent was located at “the bottom of the ocean” is now widely rejected. Miller appears to be unaware of this. In fact, as far back as 1988, American chemist Stanley Miller, who became famous when he carried out the Miller-Urey experiment in 1952, had pointed out that long-chain molecules such as RNA and proteins cannot form in water without enzymes to help them. Additionally, leading origin-of-life researchers such as Dr. John Sutherland (of the Laboratory of Molecular Biology in Cambridge, UK) and Dr. Jack Szostak (of Harvard Medical School) have discovered that many of the chemical reactions leading to life depend heavily on the presence of ultraviolet light, which only comes from the sun. This rules out a deep-sea vent scenario. That’s old news. Let us now turn to Dr. Miller’s three objections to the idea of life originating in a non-equilibrium dissipative system.
(a) Could a non-equilibrium be kept away from equilibrium?
Miller’s first objection is that non-equilibrium systems can’t be maintained for very long, which would mean that life would never have had time to form in the first place. However, a recent BBC article by Michael Marshall, titled, The secret of how life on Earth began (October 31, 2016), describes a scenario, proposed by origin-of-life researcher John Sutherland, which would evade the force of this objection. Life, Sutherland believes, may have formed very rapidly:
Sutherland has set out to find a “Goldilocks chemistry”: one that is not so messy that it becomes useless, but also not so simple that it is limited in what it can do. Get the mixture just complicated enough and all the components of life might form at once, then come together.
In other words, four billion years ago there was a pond on the Earth. It sat there for years until the mix of chemicals was just right. Then, perhaps within minutes, the first cell came into existence.
“But how, and where?” readers might ask. One plausible site for this event, put forward by origin-of-life expert Armen Mulkidjanian, is the geothermal ponds found near active volcanoes. Because these ponds would be continually receiving heat from volcanoes, they would not return to equilibrium at night, as Dr. Miller supposes.
Another likely location for the formation of life, proposed by John Sutherland, is a meteorite impact zone. This scenario also circumvents Miller’s first objection, as large-scale meteorite impacts would have melted the Earth’s crust, leading to geothermal activity and a continual supply of hot water. The primordial Earth was pounded by meteorites on a regular basis, and large impacts could have created volcanic ponds where life might have formed:
Sutherland imagines small rivers and streams trickling down the slopes of an impact crater, leaching cyanide-based chemicals from the rocks while ultraviolet radiation pours down from above. Each stream would have a slightly different mix of chemicals, so different reactions would happen and a whole host of organic chemicals would be produced.
Finally the streams would flow into a volcanic pond at the bottom of the crater. It could have been in a pond like this that all the pieces came together and the first protocells formed.
(b) Would an input of energy increase entropy?
What about Miller’s second objection, that the input of energy would increase the entropy of the system, thus making it harder for the complex chemical structures needed for life to form, in the first place?
Here, once again, recent work in the field seems to be pointing to a diametrically opposite conclusion. A recent editorial on non-equilibrium dissipative systems (Nature Nanotechnology 10, 909 (2015)) discusses the pioneering work of Dr. Jeremy England, who maintains that the dissipation of heat in these systems can lead to the self-organization of complex systems, including cells:
The theoretical concepts presented are not new — they have been rigorously reported before in specialized physics literature — but, as England explains, recently there has been a number of theoretical advances that, taken together, might lead towards a more complete understanding of non-equilibrium phenomena. More specifically, the meaning of irreversibility in terms of the amount of work being dissipated as heat as a system moves on a particular trajectory between two states. It turns out, this principle is relatively general, and can be used to explain the self-organization of complex systems such as that observed in nanoscale assemblies, or even in cells.
Dr. England, who is by the way an Orthodox Jew, has derived a generalization of the second law of thermodynamics that holds for systems of particles that are strongly driven by an external energy source, and that can dump heat into a surrounding bath. All living things meet these two criteria. Dr. England has shown that over the course of time, the more likely evolutionary outcomes will tend to be the ones that absorbed and dissipated more energy from the environment’s external energy sources, on the way to getting there. In his own words: “This means clumps of atoms surrounded by a bath at some temperature, like the atmosphere or the ocean, should tend over time to arrange themselves to resonate better and better with the sources of mechanical, electromagnetic or chemical work in their environments.”
There are two mechanisms by which a system might dissipate an increasing amount of energy over time. One such mechanism is self-replication; the other, greater structural self-organization. Natalie Wolchover handily summarizes Dr. England’s reasoning in an article in Quanta magazine, titled, A New Physics Theory of Life (January 22, 2014):
Self-replication (or reproduction, in biological terms), the process that drives the evolution of life on Earth, is one such mechanism by which a system might dissipate an increasing amount of energy over time. As England put it, “A great way of dissipating more is to make more copies of yourself.” In a September paper in the Journal of Chemical Physics, he reported the theoretical minimum amount of dissipation that can occur during the self-replication of RNA molecules and bacterial cells, and showed that it is very close to the actual amounts these systems dissipate when replicating. He also showed that RNA, the nucleic acid that many scientists believe served as the precursor to DNA-based life, is a particularly cheap building material. Once RNA arose, he argues, its “Darwinian takeover” was perhaps not surprising…
Besides self-replication, greater structural organization is another means by which strongly driven systems ramp up their ability to dissipate energy. A plant, for example, is much better at capturing and routing solar energy through itself than an unstructured heap of carbon atoms. Thus, England argues that under certain conditions, matter will spontaneously self-organize. This tendency could account for the internal order of living things and of many inanimate structures as well. “Snowflakes, sand dunes and turbulent vortices all have in common that they are strikingly patterned structures that emerge in many-particle systems driven by some dissipative process,” he said.
Dr. Jeremy England is an MIT physicist. Dr. Miller is also a physicist. I find it puzzling (and more than a little amusing) that Dr. Miller believes that increasing the entropy of a non-equilibrium dissipative system will inhibit the formation of complex chemical structures required for life to exist, whereas Dr. England believes the exact opposite!
(c) Would local variations within non-equilibrium dissipative systems prevent life from forming?
What of Dr. Miller’s third objection, that differences in temperature, concentrations, and other variables arising within a non-equilibrium dissipative system will generate entropy on a microscopic scale, creating disturbances which move the system away from a reduced-entropy state, which is required for life to exist? Dr. David Ruelle, author of a recent Arxiv paper titled, The Origin of Life seen from the point of view of non-equilibrium statistical mechanics (January 29, 2017), appears to hold a contrary view. In his paper, Dr. Ruelle refrains from proposing a specific scenario for the origin of life; rather, his aim, as he puts it, is to describe “a few plausible steps leading to more and more complex states of pre-metabolic systems so that something like life may naturally arise.” Building on the work of Dr. Jeremy England and other authors in the field, Dr. Ruelle contends that “organized metabolism and replication of information” can spontaneously arise from “a liquid bath (water containing various solutes) interacting with some pre-metabolic systems,” where the pre-metabolic systems are defined as “chemical associations which may be carried by particles floating in the liquid, or contained in cracks of a solid boundary of the liquid.” At the end of his paper, Dr. Ruelle discusses what he calls pre-biological systems, emphasizing their ability to remain stable in the face of minor fluctuations, and describing how their complexity can increase over time:
In brief, a pre-biological state is generally indecomposable. This means in particular that the fluctuations in its composition are not large. The prebiological state is also stable under small perturbations, except when those lead to a new metabolic pathway, changing the nature of the state.
We see thus a pre-biological system as a set of components undergoing an organized set of chemical reactions using a limited amount of nutrients in the surrounding fluid. The complexity of the pre-biological system increases as the amount of available nutrients decreases. The system can sustain a limited amount of disturbance. An excessive level of disturbance destroys the organized set of reactions on which the system is based: it dies.
Evidently Dr. Ruelle believes that non-equilibrium dissipative systems are resilient to minor fluctuations, and even capable of undergoing an increase in complexity, whereas Dr. Miller maintains that these fluctuations would prevent the formation of life.
If Dr. Miller believes that Dr. Ruelle is wrong, perhaps he would care to explain why.
2. The Origin of Life, Self-Organization, and Information
The three main structures phospholipids form spontaneously in solution: the liposome (a closed bilayer), the micelle and the bilayer. Image courtesy of Lady of Hats and Wikipedia.
I turn now to Dr. Miller’s second article, The Origin of Life, Self-Organization, and Information. In this article, Dr. Miller argues that there are profound differences between self-organizational order and the cellular order found in living organisms. Non-equilibrium dissipative systems might be able to generate the former kind of order, but not the latter.
The main reason for the differences between self-organizational and cellular order is that the driving tendencies in non-equilibrium systems move in the opposite direction to what is needed for both the origin and maintenance of life. First, all realistic experiments on the genesis of life’s building blocks produce most of the needed molecules in very small concentrations, if at all. And, they are mixed together with contaminants, which would hinder the next stages of cell formation. Nature would have needed to spontaneously concentrate and purify life’s precursors…
Concentration of some of life’s precursors could have taken place in an evaporating pool, but the contamination problem would then become much worse since precursors would be greatly outnumbered by contaminants. Moreover, the next stages of forming a cell would require the concentrated chemicals to dissolve back into some larger body of water, since different precursors would have had to form in different locations with starkly different initial conditions…
In addition, many of life’s building blocks come in both right and left-handed versions, which are mirror opposites. Both forms are produced in all realistic experiments in equal proportions, but life can only use one of them: in today’s life, left-handed amino acids and right-handed sugars. The origin of life would have required one form to become increasingly dominant, but nature would drive a mixture of the two forms toward equal percentages, the opposite direction.
(a) Why origin-of-life theorists are no longer obsessed with purity
Dr. Miller’s contention that contaminants would hinder the formation of living organisms has been abandoned by modern origin-of-life researchers, as BBC journalist Michael Marshall reports in his 2016 article, The secret of how life on Earth began. After describing how researcher John Sutherland was able to successfully assemble a nucleotide using a messy solution that contained a contaminant (phosphate) at the outset, leading Sutherland to hypothesize that for life to originate, there has to be “an optimum level of mess” (neither too much nor too little), Marshall goes on to discuss the pioneering experiments of another origin-of-life researcher, Jack Szostak, whose work has led him to espouse the same conclusion as Sutherland:
Szostak now suspects that most attempts to make the molecules of life, and to assemble them into living cells, have failed for the same reason: the experiments were too clean.
The scientists used the handful of chemicals they were interested in, and left out all the other ones that were probably present on the early Earth. But Sutherland’s work shows that, by adding a few more chemicals to the mix, more complex phenomena can be created.
Szostak experienced this for himself in 2005, when he was trying to get his protocells to host an RNA enzyme. The enzyme needed magnesium, which destroyed the protocells’ membranes.
The solution was a surprising one. Instead of making the vesicles out of one pure fatty acid, they made them from a mixture of two. These new, impure vesicles could cope with the magnesium – and that meant they could play host to working RNA enzymes.
What’s more, Szostak says the first genes might also have embraced messiness.
Modern organisms use pure DNA to carry their genes, but pure DNA probably did not exist at first. There would have been a mixture of RNA nucleotides and DNA nucleotides.
In 2012 Szostak showed that such a mixture could assemble into “mosaic” molecules that looked and behaved pretty much like pure RNA. These jumbled RNA/DNA chains could even fold up neatly.
This suggested that it did not matter if the first organisms could not make pure RNA, or pure DNA…
(b) Getting it together: how the first cell may have formed
Dr. Miller also argues that even if the components of life were able to form in separate little pools, the problem of how they came to be integrated into a living cell would still remain. In recent years, Dr. John Sutherland has done some serious work on this problem. Sutherland describes his own preferred origin-of-life scenario in considerable detail in a paper titled, The Origin of Life — Out of the Blue (Angewandte Chemie, International Edition, 2016, 55, 104 – 121.
Several years ago, we realised that … polarisation of the field was severely hindering progress, and we planned a more holistic approach. We set out to use experimental chemistry to address two questions, the previously assumed answers to which had led to the polarisation of the field: “Are completely different chemistries needed to make the various subsystems?” [and] “Would these chemistries be compatible with each other?”…
With twelve amino acids, two ribonucleotides and the hydrophilic moiety of lipids synthesised by common chemistry, we feel that we have gone a good way to answering the first of the questions we posed at the outset. “Are completely different chemistries needed to make the various subsystems?” — we would argue no! We need to find ways of making the purine ribonucleotides, but hydrogen cyanide 1 is already strongly implicated as a starting material. We also need to find ways of making the hydrophobic chains of lipids, and maybe a few other amino acids, but there is hope in reductive homologation chemistry or what we have called “cyanosulfidic protometabolism.” …
The answer to the second question — “Would these chemistries be compatible with each other?” — is a bit more vague (thus far). The chemistries associated with the different subsystems are variations on a theme, but to operate most efficiently some sort of separation would seem to be needed. Because a late stage of our scenario has small streams or rivulets flowing over ground sequentially leaching salts and other compounds as they are encountered (Figure 17), it provides a very simple way in which variants of the chemistry could play out separately before all the products became mixed.
Separate streams might encounter salts and other compounds in different orders and be exposed to solar radiation differently. Furthermore streams might dry out and the residues become heated through geothermal activity before fresh inflow of water. If streams with different flow chemistry histories then merged, convergent synthesis might occur at the confluence and downstream thereof, or products might simply mix. It would be most plausible if only a few streams were necessary for the various strands of the chemistry to operate efficiently before merger. Our current working model divides the reaction network up such that the following groups of building blocks would be made separately: ribonucleotides; alanine, threonine, serine and glycine; glycerol phosphates, valine and leucine; aspartic acid, asparagine, glutamic acid and glutamine; and arginine and proline. Because the homologation of all intermediates uses hydrogen cyanide (1), products of reductive homologation of (1) — especially glycine — could be omnipresent.
Since then, Sutherland has written another paper, titled, Opinion: Studies on the origin of life — the end of the beginning (Nature Reviews Chemistry 1, article number: 0012 (2017), doi:10.1038/s41570-016-0012), in which he further develops his scenario.
(c) How did life come to be left-handed?
Two enantiomers [mirror images] of a generic amino acid which is chiral [left- or right-handed]. Image courtesy of Wikipedia.
The left-handedness of amino acids in living things, coupled with the fact that that organisms contain only right-handed nucleotides, has puzzled origin-of-life theorists since the time of Pasteur. Does this rule put a natural origin for living things, as Dr. Miller thinks? It is interesting to note that Pasteur himself was wary of drawing this conclusion, according to a biography written by his grandson, and he even wrote: “I do not judge it impossible.” (René Dubos, Louis Pasteur: Free Lance of Science, Da Capo Press, Inc., 1950. p 396.)
Pasteur’s caution turns out to have been justified. According to a 2015 report by Claudia Lutz in Phys.org titled, Straight up, with a twist: New model derives homochirality from basic life requirements, scientists at the University of Illinois have recently come up with a simple model which explains the chirality found in living organisms in terms of just two basic properties: self-replication and disequilibrium.
The Illinois team wanted to develop a … model, … based on only the most basic properties of life: self-replication and disequilibrium. They showed that with only these minimal requirements, homochirality appears when self-replication is efficient enough.
The model … takes into account the chance events involving individual molecules — which chiral self-replicator happens to find its next substrate first. The detailed statistics built into the model reveal that if self-replication is occurring efficiently enough, this incidental advantage can grow into dominance of one chirality over the other.
The work leads to a key conclusion: since homochirality depends only on the basic principles of life, it is expected to appear wherever life emerges, regardless of the surrounding conditions.
More recent work has provided a detailed picture of how life’s amino acids became left-handed. In 2016, Rowena Ball and John Brindley proposed that since hydrogen peroxide is the smallest, simplest molecule to exist as a pair of non-superimposable mirror images, or enantiomers, its interactions with ribonucelic acids may have led to amplification of D-ribonucleic acids and extinction of L-ribonucleic acids. Hydrogen peroxide was produced on the ancient Earth, more than 3.8 billion years ago, around the time that life emerged. Ball explains how this favored the emergence of right-handed nucleotide chains, in a recent article in Phys.org:
It is thought that a small excess of L-amino acids was “rained” onto the ancient Earth by meteorite bombardment, and scientists have found that a small excess of L-amino acids can catalyse formation of small excesses of D-nucleotide precursors. This, we proposed, led to a marginal excess of D-polynucleotides over L-polynucleotides, and a bias to D-chains of longer mean length than L-chains in the RNA world.
In the primordial soup, local excesses of one or other hydrogen peroxide enantiomer would have occurred. Specific interactions with polynucleotides destabilise the shorter L-chains more than the longer, more robust, D-chains. With a greater fraction of L-chains over D-chains destabilised, hydrogen peroxide can then “go in for the kill”, with one enantiomer (let us say M) preferentially oxidising L-chains.
Overall, this process works in favour of increasing the fraction and average length of D-chains at the expense of L-species.
An outdated argument relating to proteins
In his article, Dr. Miller puts forward an argument for proteins having been intelligently designed, which unfortunately rests on faulty premises:
Proteins eventually break down, and they cannot self-replicate. Additional machinery was also needed to constantly produce new protein replacements. Also, the proteins’ sequence information had to have been stored in DNA using some genetic code, where each amino acid was represented by a series of three nucleotides know as a codon in the same way English letters are represented in Morse Code by dots and dashes. However, no identifiable physical connection exists between individual amino acids and their respective codons. In particular, no amino acid (e.g., valine) is much more strongly attracted to any particular codon (e.g., GTT) than to any other. Without such a physical connection, no purely materialistic process could plausibly explain how amino acid sequences were encoded into DNA. Therefore, the same information in proteins and in DNA must have been encoded separately.
The problem with this argument is that scientists have known for decades that its key premise is false. Dennis Venema (Professor of Biology at Trinity Western University in Langley, British Columbia) explains why, in a Biologos article titled, Biological Information and Intelligent Design: Abiogenesis and the origins of the genetic code (August 25, 2016):
Several amino acids do in fact directly bind to their codon (or in some cases, their anticodon), and the evidence for this has been known since the late 1980s in some cases. Our current understanding is that this applies only to a subset of the 20 amino acids found in present-day proteins.
In Venema’s view, this finding lends support to a particular hypothesis about the origin of the genetic code, known as the direct templating hypothesis, which proposes that “the tRNA system is a later addition to a system that originally used direct chemical interactions between amino acids and codons.” Venema continues: “In this model, then, the original code used a subset of amino acids in the current code, and assembled proteins directly on mRNA molecules without tRNAs present. Later, tRNAs would be added to the system, allowing for other amino acids—amino acids that cannot directly bind mRNA — to be added to the code.” The model also makes a specific prediction: if it is correct, then “amino acids would directly bind to their codons on mRNA, and then be joined together by a ribozyme (the ancestor of the present-day ribosome).”
Venema concludes:
The fact that several amino acids do in fact bind their codons or anticodons is strong evidence that at least part of the code was formed through chemical interactions — and, contra [ID advocate Stephen] Meyer, is not an arbitrary code. The code we have — or at least for those amino acids for which direct binding was possible — was indeed a chemically favored code. And if it was chemically favored, then it is quite likely that it had a chemical origin, even if we do not yet understand all the details of how it came to be.
I will let readers draw their own conclusions as to who has the better of the argument here: Venema or Miller.
3. Free Energy and the Origin of Life: Natural Engines to the Rescue

Photograph of American scientist Josiah Willard Gibbs (1839-1903), discoverer of Gibbs free energy, taken about 1895. Image courtesy of Wikipedia.
In his third article, Free Energy and the Origin of Life: Natural Engines to the Rescue, Dr. Miller argues that the emergence of life would have required chemicals to move from a state of high entropy and low free energy to one of low entropy and high free energy. (Gibbs free energy can be defined as “a thermodynamic potential that can be used to calculate the maximum of reversible work that may be performed by a thermodynamic system at a constant temperature and pressure.”) However, spontaneous natural processes always tend towards lower free energy. An external source of energy doesn’t help matters, either, as it increases entropy. Miller concludes that life’s formation was intelligently directed:
Now, I will address attempts to overcome the free-energy barriers through the use of natural engines. To summarize, a fundamental hurdle facing all origin-of-life theories is the fact that the first cell must have had a free energy far greater than its chemical precursors. And spontaneous processes always move from higher free energy to lower free energy. More specifically, the origin of life required basic chemicals to coalesce into a state of both lower entropy and higher energy, and no such transitions ever occur without outside help in any situation, even at the microscopic level.
Attempted solutions involving external energy sources fail since the input of raw energy actually increases the entropy of the system, moving it in the wrong direction. This challenge also applies to all appeals to self-replicating molecules, auto-catalytic chemical systems, and self-organization. Since all of these processes proceed spontaneously, they all move from higher to lower free energy, much like rocks rolling down a mountain. However, life resides at the top of the mountain. The only possible solutions must assume the existence of machinery that processes energy and directs it toward performing the required work to properly organize and maintain the first cell.
But as we saw above, Dr. Jeremy England maintains that increasing the entropy of a non-equilibrium dissipative system can promote the formation of complex chemical structures required for life to exist. Miller’s claim that external energy sources invariably increase the entropy of the system is correct if we consider the system as a whole, including the bath into which non-equilibrium dissipative systems can dump their heat. Internally, however, self-organization can reduce entropy- a fact which undermines Miller’s attempt to demonstrate the impossibility of abiogenesis.
4. Origin of Life and Information — Some Common Myths

A game of English-language Scrabble in progress. Image courtesy of Wikipedia.
In his final installment, Origin of Life and Information — Some Common Myths, Dr. Miller takes aim at the view that a reduction in entropy is sufficient to account for the origin of biological information. Miller puts forward three arguments which he believes demonstrate the absurdity of this idea.
(a) Could a reduction in entropy generate functional information?

A bowl of alphabet soup nearly full, and nearly empty, spelling “THE END” in the latter case. Image courtesy of strawberryblues and Wikipedia.
Let’s look at Miller’s first argument, that a reduction in entropy could never account for the highly specific sequencing of amino acids in proteins, or nucleotides in DNA:
A common attempt to overcome the need for information in the first cell is to equate information to a reduction in entropy, often referred to as the production of “negative entropy” or N-entropy. … However, entropy is not equivalent to the information in cells, since the latter represents functional information. To illustrate the difference, imagine entering the kitchen and seeing a bowl of alphabet soup with several letters arranged in the middle as follows:
REST TODAY AND DRINK PLENTY OF FLUIDS
I HOPE YOU FEEL BETTER SOON
You would immediately realize that some intelligence, probably your mother, arranged the letters for a purpose. Their sequence could not possibly be explained by the physics of boiling water or the chemistry of the pasta.
… You would immediately recognize that a reduction in thermal entropy has no physical connection to the specific ordering of letters in a meaningful message. The same principle holds true in relation to the origin of life for the required sequencing of amino acids in proteins or nucleotides in DNA.
This, I have to say, is a fallacious argument, which I refuted in my online review of Dr. Douglas Axe’s recently published book, Undeniable. Dr. Miller, like Dr. Axe, is confusing functional information (which is found in living things) with the semantic information found in a message written with the letters of the alphabet, such as “REST TODAY AND DRINK PLENTY OF FLUIDS.” In fact, functional information is much easier to generate than semantic information, because it doesn’t have to form words, conform to the rules of syntax, or make sense at the semantic level:
The concepts of meaning and function are quite different, for reasons I shall now explain.
In order for an accidentally generated string of letters to convey a meaningful message, it needs to satisfy three very stringent conditions, each more difficult than the last: first, the letters need to be arranged into meaningful words; second, the sequence of words has to conform to the rules of syntax; and finally, the sequence of words has to make sense at the semantic level: in other words, it needs to express a meaningful proposition. For a string of letters generated at random to meet all of these conditions would indeed be fantastically improbable. But here’s the thing: living things don’t need to satisfy any of these conditions... The sequence of amino acids in a protein needs to do just one thing: it needs to fold up into a shape that can perform a biologically useful task. And that’s it. Generating something useful by chance – especially something with enough useful functions to be called alive – is a pretty tall order, but because living things lack the extra dimensions of richness found in messages that carry a semantic meaning, they’re going to be a lot easier to generate by chance than (say) instruction manuals or cook books… In practical terms, that means that given enough time, life just might arise.
Let me be clear: I am not trying to argue that a reduction in entropy is sufficient to account for the origin of biological information. That strikes me as highly unlikely. What I am arguing, however, is that appeals to messages written in text are utterly irrelevant to the question of how biological information arose. I might also add that most origin-of-life theorists don’t believe that the first living things contained highly specific sequences of “amino acids in proteins or nucleotides in DNA,” as Miller apparently thinks, because they probably lacked both proteins and DNA, if the RNA World hypothesis (discussed below) is correct. Miller is attacking a straw man.
(b) Can fixed rules account for the amino acid sequencing in proteins?
Dr. Miller’s second argument is that fixed rules, such as those governing non-linear dynamics processes, would be unable to generate the arbitrary sequences of amino acids found in proteins. These sequences perform useful biological functions, but are statistically random:
A related error is the claim that biological information could have come about by some complex systems or non-linear dynamics processes. The problem is that all such processes are driven by physical laws or fixed rules. And, any medium capable of containing information (e.g., Scrabble tiles lined up on a board) cannot constrain in any way the arrangement of the associated symbols/letters. For instance, to type a message on a computer, one must be free to enter any letters in any order. If every time one typed an “a” the computer automatically generated a “b,” the computer could no longer contain the information required to create meaningful sentences. In the same way, amino acid sequences in the first cell could only form functional proteins if they were free to take on any order…
…To reiterate, no natural process could have directed the amino acid sequencing in the first cell without destroying the chains’ capacity to contain the required information for proper protein folding. Therefore, the sequences could never be explained by any natural process but only by the intended goal of forming the needed proteins for the cell’s operations (i.e., teleologically).
Dr. Miller has a valid point here: it is extremely unlikely that fixed rules, by themselves, can explain the origin of biological information. Unfortunately, he spoils his case by likening the information in a protein to a string of text. As we have seen, the metaphor is a flawed one, on three counts. Proteins contain functional information, not semantic information.
Dr. Miller also makes an illicit inference from the statement that “no natural process could have directed the amino acid sequencing in the first cell” to the conclusion that “the sequences could never be explained by any natural process,” but only by a teleological process of Intelligent Design. This inference is unwarranted on two counts. First, it assumes that the only kind of natural explanation for the amino acid sequences in proteins would have to be some set of fixed rules (or laws) directing their sequence, which overlooks the possibility that functional sequences may have arisen by chance. (At this point, Intelligent Design advocates will be sure to cite Dr. Douglas Axe’s estimate that only 1 in 10^77 sequences of 150 amino acids are capable of folding up and performing some useful biological function, but this figure is a myth.)
Second, teleology may be either intrinsic (e.g. hearts are of benefit to animals, by virtue of the fact that they pump blood around the body) or extrinsic (e.g. a machine which is designed for the benefit of its maker), or both. Even if one could show that a teleological process was required to explain the origin of proteins, it still needs to be shown that this process was designed by an external Intelligence.
(c) Can stereochemical affinity account for the origin of the genetic code?

Origin-of-life researcher Eugene Koonin in May 2013. Koonin is a Russian-American biologist and Senior Investigator at the National Center for Biotechnology Information (NCBI). Image courtesy of Konrad Foerstner and Wikipedia.
Dr. Miller then goes on to criticize the stereochemical affinity hypothesis for the origin of the genetic code, citing the work of Dr. Eugene Koonin:
A third error relates to attempts to explain the genetic code in the first cell by a stereochemical affinity between amino acids and their corresponding codons. According to this model, naturally occurring chemical processes formed the basis for the connection between amino acids and their related codons (nucleotide triplets). Much of the key research promoting this theory was conducted by biochemist Michael Yarus. He also devised theories on how this early stereochemical era could have evolved into the modern translation system using ribosomes, tRNAs, and supporting enzymes. His research and theories are clever, but his conclusions face numerous challenges.
…For instance, Andrew Ellington’s team questioned whether the correlations in these studies were statistically significant, and they argued that his theories for the development of the modern translation system were untenable. Similarly, Eugene Koonin found that the claimed affinities were weak at best and generally unconvincing. He argued instead that the code started as a “frozen accident” undirected by any chemical properties of its physical components.
I should point out here that some of the articles which Miller links to here are rather old. For example, the article by Andrew Ellington’s team questioning the statistical significance of the correlations identified by Yarus dates back to 2000, while Koonin’s critique dates back to 2008. This is significant, as Yarus et al. published an article in 2009 presenting some of their strongest statistical evidence for the stereochemical model they were proposing:
Using recent sequences for 337 independent binding sites directed to 8 amino acids and containing 18,551 nucleotides in all, we show a highly robust connection between amino acids and cognate coding triplets within their RNA binding sites. The apparent probability (P) that cognate triplets around these sites are unrelated to binding sites is [about] 5.3 x 10-45 for codons overall, and P [is about] 2.1 x 10-46 for cognate anticodons. Therefore, some triplets are unequivocally localized near their present amino acids. Accordingly, there was likely a stereochemical era during evolution of the genetic code, relying on chemical interactions between amino acids and the tertiary structures of RNA binding sites. (Michael Yarus, Jeremy Joseph Widmann and Rob Knight, “RNA–Amino Acid Binding: A Stereochemical Era for the Genetic Code,” in Journal of Molecular Evolution, November 2009; 69(5):406-29, DOI 10.1007/s00239-009-9270-1.)
Dr. Miller also neglects to mention that while Dr. Eugene Koonin did indeed critique Yarus’ claims in the more recent 2017 article he linked to, Koonin actually proposed his own variant of the stereochemical model for the origin of the genetic code:
The conclusion that the mRNA decoding in the early translation system was performed by RNA molecules, conceivably, evolutionary precursors of modern tRNAs (proto-tRNAs) [89], implies a stereochemical model of code origin and evolution, but one that differs from the traditional models of this type in an important way (Figure 2). Under this model, the proto-RNA-amino acid interactions that defined the specificity of translation would not involve the anticodon (let alone codon) that therefore could be chosen arbitrarily and fixed through frozen accident. Instead, following the reasoning outlined previously [90], the amino acids would be recognized by unique pockets in the tertiary structure of the proto-tRNAs. The clustering of codons for related amino acids naturally follows from code expansion by duplication of the proto-tRNAs; the molecules resulting from such duplications obviously would be structurally similar and accordingly would bind similar amino acids, resulting in error minimization, in accord with Crick’s proposal (Figure 2).
Apparently, the reason why Koonin considers that “attempts to decipher the primordial stereochemical code by comparative analysis of modern translation system components are largely futile” is that “[o]nce the amino acid specificity determinants shifted from the proto-tRNAs to the aaRS, the amino acid-binding pockets in the (proto) tRNAs deteriorated such that modern tRNAs showed no consistent affinity to the cognate amino acids.” Koonin proposes that “experiments on in vitro evolution of specific aminoacylating ribozymes that can be evolved quite easily and themselves seem to recapitulate a key aspect of the primordial translation system” might help scientists to reconstruct the original code, at some future date.
Although (as Dr. Miller correctly notes) Koonin personally favors the frozen accident theory for the origin of the genetic code, he irenically proposes that “stereochemistry, biochemical coevolution, and selection for error minimization could have contributed synergistically at different stages of the evolution of the code [43] — along with frozen accident.”
Positive evidence against the design of the genetic code
But there is much more to Koonin’s article. Koonin presents damning evidence against the hypothesis that the standard genetic code (or SGC) was designed, in his article. The problem is that despite the SGC’s impressive ability to keep the number of mutational and translational errors very low, there are lots of other genetic codes which are even better:
Extensive quantitative analyses that employed cost functions differently derived from physico-chemical properties of amino acids have shown that the code is indeed highly resilient, with the probability to pick an equally robust random code being on the order of 10−7–10−8 [14,15,61,62,63,64,65,66,67,68]. Obviously, however, among the ~1084 possible random codes, there is a huge number with a higher degree of error minimization than the SGC [standard genetic code – VJT]. Furthermore, the SGC is not a local peak on the code fitness landscape because certain local rearrangements can increase the level of error minimization; quantitatively, the SGC is positioned roughly halfway from an average random code to the summit of the corresponding local peak [15] (Figure 1).
Let’s do the math. There are about 1084 possible genetic codes. The one used by living things is in the top 1 in 100 million (or 1 in 108). That means that there are 1076 possible genetic codes that are better than it. To make matters worse, it’s not even the best code in its local neighborhood. It’s not “on top of a hill,” as it were. It’s about half-way up the hill. Now ask yourself: if the genetic code were intelligently designed, is this a result that one would expect?
It is disappointing that Dr. Miller fails to appreciate the significance of this evidence against design, presented by Koonin. Sadly, he never even mentions it in his article.
The RNA World – fatally flawed?
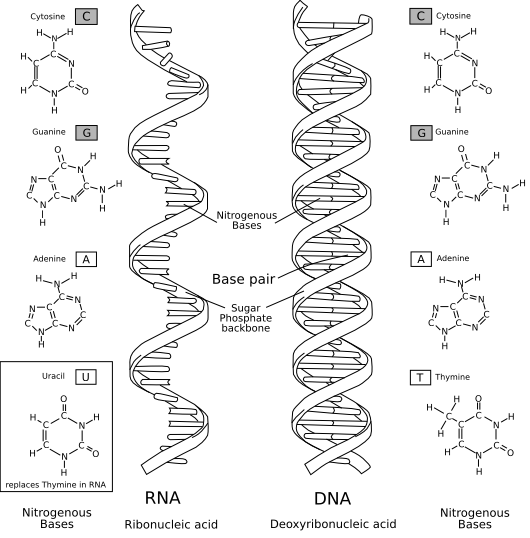
A comparison of RNA (left) with DNA (right), showing the helices and nucleobases each employs. Image courtesy of Access Excellence and Wikipedia.
Finally, Dr. Miller concludes with a number of critical remarks about the RNA world hypothesis. Before we proceed further, a definition of the hypothesis might be in order:
All RNA World hypotheses include three basic assumptions: (1) At some time in the evolution of life, genetic continuity was assured by the replication of RNA; (2) Watson-Crick base-pairing was the key to replication; (3) genetically encoded proteins were not involved as catalysts. RNA World hypotheses differ in what they assume about life that may have preceded the RNA World, about the metabolic complexity of the RNA World, and about the role of small-molecule cofactors, possibly including peptides, in the chemistry of the RNA World.
(Michael P. Robertson and Gerald F. Joyce, The Origins of the RNA World, Cold Spring Harbor Perspectives in Biology, May 2012; 4(5): a003608.)
In the article cited above, Robertson and Joyce summarize the evidence for the RNA World:
There is now strong evidence indicating that an RNA World did indeed exist on the early Earth. The smoking gun is seen in the structure of the contemporary ribosome (Ban et al. 2000; Wimberly et al. 2000; Yusupov et al. 2001). The active site for peptide-bond formation lies deep within a central core of RNA, whereas proteins decorate the outside of this RNA core and insert narrow fingers into it. No amino acid side chain comes within 18 Å of the active site (Nissen et al. 2000). Clearly, the ribosome is a ribozyme (Steitz and Moore 2003), and it is hard to avoid the conclusion that, as suggested by Crick, “the primitive ribosome could have been made entirely of RNA” (1968).
A stronger version of the RNA World hypothesis is that life on Earth began with RNA. In the article cited above, Robertson and Joyce are very frank about the difficulties attending this hypothesis, even referring to it as “The Prebiotic Chemist’s Nightmare.” Despite their sympathies for this hypothesis, the authors suggest that it may be fruitful to consider “the alternative possibility that RNA was preceded by some other replicating, evolving molecule, just as DNA and proteins were preceded by RNA.”
The RNA World hypothesis has its scientific advocates and critics. In a recent article in Biology Direct, Harold S. Bernhardt describes it as “the worst theory of the early evolution of life (except for all the others),” in a humorous adaptation of Sir Winston Churchill’s famous comment on democracy. Referee Eugene Koonin agrees, noting that “no one has achieved bona fide self-replication of RNA which is the cornerstone of the RNA World,” but adding that “there is a lot going for the RNA World … whereas the other hypotheses on the origin of life are outright helpless.” Koonin continues: “As Bernhardt rightly points out, it is not certain that RNA was the first replicator but it does seem certain that it was the first ‘good’ replicator.”
In 2009, Gerald Joyce and Tracey Lincoln of the Scripps Research Institute in La Jolla, California, managed to create an RNA enzyme that replicates itself indirectly, by joining together two short pieces of RNA to create a second enzyme, which then joins together another two RNA pieces to recreate the original enzyme. Although the cycle was capable of being continued indefinitely, given an input of suitable raw materials, the enzymes were only able to do their job if they were given the correct RNA strands, which Joyce and Lincoln had to synthesize.
The RNA world hypothesis received a further boost in March 2015, when NASA scientists announced for the first time that, using the starting chemical pyrimidine, which is found in meteorites, they had managed to recreate three key components of DNA and RNA: uracil, cytosine and thymine. The scientists used an ice sample containing pyrimidine exposed to ultraviolet radiation under space-like conditions, in order to produce these essential ingredients of life. “We have demonstrated for the first time that we can make uracil, cytosine, and thymine, all three components of RNA and DNA, non-biologically in a laboratory under conditions found in space,” said Michel Nuevo, research scientist at NASA’s Ames Research Center, Moffett Field, California. “We are showing that these laboratory processes, which simulate conditions in outer space, can make several fundamental building blocks used by living organisms on Earth.”
As if that were not enough, more good news for the hypothesis emerged in 2016. RNA is composed of four different chemical building blocks: adenine (A), guanine (G), cytosine (C), and uracil (U). Back in 2009, a team of researchers led by John Sutherland showed a plausible series of chemical steps which might have given rise to cytosine and uracil, which are also known as pyrimidines, on the primordial Earth.However, Sutherland’s team wasn’t able to explain the origin of RNA’s purine building blocks, adenine and guanine. At last, a team of chemists led by Thomas Carell, at Ludwig Maximilian University of Munich in Germany, has filled in this gap in scientists’ knowledge. They’ve found a synthetic route for making purines. To be sure, problems remain, as reporter Robert Service notes in a recent article in Science magazine (‘RNA world’ inches closer to explaining origins of life, May 12, 2016):
…Steven Benner, a chemist and origin of life expert at the Foundation for Applied Molecular Evolution in Alachua, Florida… agrees that the newly suggested purine synthesis is a “major step forward” for the field. But even if it’s correct, he says, the chemical conditions that gave rise to the purines still don’t match those that Sutherland’s group suggests may have led to the pyrimidines. So just how As, Gs, Cs, and Us would have ended up together isn’t yet clear. And even if all the RNA bases were in the same place at the same time, it’s still not obvious what drove the bases to link up to form full-fledged RNAs, Benner says.
I conclude that whatever difficulties attend the RNA World hypothesis, they are not fatal ones. Miller’s case for the intelligent design of life is far from closed.
Conclusion

James M. Tour is a synthetic organic chemist, specializing in nanotechnology. Dr. Tour is the T. T. and W. F. Chao Professor of Chemistry, Professor of Materials Science and NanoEngineering, and Professor of Computer Science at Rice University in Houston, Texas. Image courtesy of Wikipedia.
At the beginning of this post, I invited my readers to consider the question of whether Dr. Miller has made a strong scientific case for Intelligent Design. Now, I would happily grant that he has highlighted a number of difficulties for any naturalistic theory of the origin of life. But I believe I have shown that Dr. Miller’s positive case for Intelligent Design consists largely of trying to put a full stop where science leaves a comma. Dr. Miller seems to have ignored recent developments in the field of origin-of-life research, which remove at least some of the difficulties he alludes to in his articles. I think an unbiased reader would have to conclude that Miller has failed to demonstrate, to even a high degree of probability, the need for a Designer of life.
I’d like to finish with two quotes from an online essay (Origin of Life, Intelligent Design, Evolution, Creation and Faith) by Dr. James Tour, a very fair-minded chemist who has written a lot about the origin of life:
I have been labeled as an Intelligent Design (sometimes called “ID”) proponent. I am not. I do not know how to use science to prove intelligent design although some others might. I am sympathetic to the arguments and I find some of them intriguing, but I prefer to be free of that intelligent design label. As a modern-day scientist, I do not know how to prove intelligent design using my most sophisticated analytical tools— the canonical tools are, by their own admission, inadequate to answer the intelligent design question. I cannot lay the issue at the doorstep of a benevolent creator or even an impersonal intelligent designer. All I can presently say is that my chemical tools do not permit my assessment of intelligent design.
and
Those who think scientists understand the issues of prebiotic chemistry are wholly misinformed. Nobody understands them. Maybe one day we will. But that day is far from today.
Amen to both.

If ENV wants to recycle bad arguments, then I’ll have to admit that they have a large pool of bad arguments that they can call on.
Well done VJ!
What a thoughtful, informative piece! Thanks very much, Vince>
Thanks VJ. I wrote Dr. Miller a letter on some of the points regarding thermodynamics.
I pointed out entropy must often be increased to enable life. For example a warm living rat has MORE entropy than a frozen dead one.
Here is something which I’d almost send to any ID proponent arguing for the 2nd law’s relevance to ID. I sent a copy to Dr. Miller. It is jumble of thoughts, but I was pressed for time:
Impressive article Vincent, I have to say.
As others have said, great article, Vincent. Thanks.
Hi Tomato Addict, walto, Sal, Rumraket and Alan,
Thank you very much for your kind words. I’m glad you all enjoyed the article.
Sal, I greatly appreciated your clarifying remarks on the concept of entropy. Too many people mistakenly equate it with disorder, and I’d like to thank you for setting the record straight.
Re the law of large numbers: I think it could be used to justify design inferences in some cases (e.g. for coin tosses, in carefully defined circumstances), but I can only imagine it being used in the case of homochirality if one could somehow show that a long chain of L-amino acids suddenly formed on the primordial Earth from a racemic mixture. That would be very fishy.
Hi Neil Rickert,
I may be writing some more posts in the future on bad arguments recycled by other contributors to ENV. Stay tuned.
The BioLogos posts on direct templating are fascinating — thanks for the links!
VJT
Dont you mean that self-organization can increase entropy? I thought that here you’re addressing the erronous view that conflates complexity with entropy. There are systems, which because of the potential for interactions among the parts, will increase in complexity as free energy dissipates and entropy increases.
VJT…… aha. As I continue reading I see you may have addressed my comment above.
Thanks very much for this post btw!!
vjtorley,
Great post VJT.
I think this argument works if translation error optimization is the only parameter that the code is meant to optimize.
Another consideration is assignment to minimize error from DNA mutation as more condons are assigned to the highest use amino acids.
Hi Kantain Naturalist, REW and colewd,
Thanks for your comments. Dennis Venema has an excellent series of articles over at Biologos, which is well worth reading:
http://biologos.org/blogs/dennis-venema-letters-to-the-duchess/biological-information-and-intelligent-design-abiogenesis-and-the-origins-of-the-genetic-code
colewd, your suggestion of an additional constraint besides error minimization sounds interesting. Well worth a post, I’d say. Cheers.
This is a complete non-sequitor from VJ (whose has time to run down every bad conclusion he has drawn?)
What is being “better” at a genetic code mean? How would we know one could be better? Better at what? We don’t have any evidence that even ONE other genetic code would work better than this one, and yet he makes this statement.
VJ’s mode of operation is to regularly just made wide sweeping assertions and hope no one notices, through his smoke screen of volume.
Actually you are right, that math is off. If the genetic code is in the top 100 million out of those 10^84 possible genetic codes, then there are at most 99 million better codes. Then the remaining 10^76 codes are worse, not better.
It is well recognized that the genetic code is one of the best possible codes. But it is not actually THE best possible code. There are still millions of superior codes.
This is where you are wrong. We know exactly what the genetic code is good at. It is good at minimizing the deleterious effects of mutations and thus, facilitate evolution without causing too many catastrophic changes in important proteins.
More precisely, the codons are so arranged that the chance of a nucleotide substitution resulting in an amino-acid substitution is significantly reduced, compared to a randomly arranged code. IIRC in general 44 of the 64 codons can be mutated four different ways without causing an amino acid substitution because four codons code for these same amino acid.
Also, the codons are so made that a nucleotide substitution that nevertheless does result in an amino acid substitution, in general will result in a substitution by an amino-acid with more similar chemical properties (such as polarity or solubility). So the overall effect on the resulting protein is reduced.
phoodoo,
It’s clear you’ve missed a big part of the general debate about this. And this stuff has been known for many many years now. I guess it’s too technical for your level of engagement with the culture wars.
Hint: Just because you don’t know something does not mean nobody knows things.
So the genetic code is not randomly arranged? What made it non-random?
Let me rephrase my last sentence there. It should read “…compared to a coding order picked at random”.
Anyway, to answer your question, the coding order of the genetic code is in part due to natural selection to reduce the deleterious effects of mutations on protein amino acid sequence.
An analysis of the ancestral functions of the aminoacyl-tRNA-synthetases reveals that they were highly promiscous (meaning they would charge tRNA with several different amino acids), which in turn entails that the first genetic code was synthesizing statistical (aka essentially random) amino acid chains. From that stage, the coding order we see today evolved.
There are other contributing factors besides natural selection. Several amino acids show chemical and stereochemical affinity to their codons (or anti codons), which would explain the assignment of particular codons to particular amino acids. This is called the stereochemical theory.
Then there’s the inferred order of evolution of biosynthetic pathways for amino acid synthesis, which in turn would make them available to use in proteins. This is called the coevolution theory.
And then there’s the fact that once a number of important proteins had evovled which in turn depended on the establishment of a code for their reliable synthesis, the code would be very unlikely to evolve further, cementing it’s “state” for all subsequent life. This last one is called the “frozen accident”. No, it doesnt’ mean the genetic code is an accident that just popped up out of nowhere, it explains why the code doesn’t change any further into even better codes for example.
These four factors all contributed to the nature of the genetic code we have. So they are (in no particular order), natural selection for “error minimization”, coevolution with biosynthetic pathways, stereochemistry and “frozen accident”. These are what explain the coding order of the genetic code we see in all life we know of.
Seems like someone is getting a free education to me. Stick around phoodoo, we’ll make you one of us yet.
Rumraket,
So you mean the random genetic code got a random mutation which made it less likely to get random mutations, and thus natural selection favored the code that got the random mutation, so it is not random anymore?
This keeps getting more fun.
phoodoo, basing an entire worldview on equivocation with respect to the word “random” is a weird life choice, IMHO.
walto,
Yes, you better let the evolutionists know this. I am not sure why they do it.
Yes, that is what I mean. Not even joking.
Well, actually not less likely to get random mutations, but less likely for those random mutations to have strongly deleterious effects. But yes that is essentially it.
I was reflecting earlier on how the ID position rests on conflating (at least) two very different senses of “randomness”: one sense from biology and another sense from mathematics.
But that’s none of my business.
phoodoo,
You may be interested in this: https://en.wikipedia.org/wiki/Muller%27s_ratchet
Guess who coined the phrase?
Some consider it a precursor to Irreducible Complexity, which I’m sure you know all about already.
I will agree that many people, including many popular scientists are very impresise with their word of the use random. It doesn’t help that there’s actually several different ways the way can be understood.
Some use “X is random” to mean “X has several outcomes with an equiprobable distribution” (An example could be a fair coin).
Some use “X is random” to mean “X has several outcomes, but they don’t need to be equiprobable to still be random” (An example could be a weighted coin that has a bias towards one side).
Some use “X is random” to mean only that “X is analyzed in terms of probability theory” (Everything for which the outcomes are expressed in terms of probabilities, like card-games or the lottery).
And then there are some few who use “X is random” to mean “X is without a known cause”.
To make matters worse, it’s often times not clear in which of these senses the word is used when it is and several of them are not mutually exclusive.
Rumraket,
That’s helpful. But the causes of phenotypic variation in a population — i.e. mutations — are not ‘random’ in any of those senses.
Sure they are. Mutations are random in both the sense that they’re analysed in terms of probability theory, and they are random but biased in their distributions.
Hi Rumraket,
You write:
“Actually you are right, that math is off. If the genetic code is in the top 100 million out of those 10^84 possible genetic codes, then there are at most 99 million better codes. Then the remaining 10^76 codes are worse, not better.”
What I wrote was not that the genetic code used by living things is in the top 100 million, but that it is is in the top 1 in 100 million (or 1 in 10^8). In other words, out of every 100 million possible codes, 1 code is better than the code we have.
10^84 divided by 100 million (or 10^8) is 10^76. My math is correct.
Think of it this way. If the standard genetic code were in the top 1 in 10 possible genetic codes, in terms of error minimization, then how many codes would be better than it? Answer: 10^84 divided by 10, or 10^83. 1 in 100 million is more selective: it’s 1 in 10^8. But it still leaves us with a “huge number” of possible codes (Koonin’s words) which are better than ours.
And here’s the real kicker: the code we have is half-way up a hill, in terms of efficiency in minimizing errors.
I don’t understand what you mean by the expression “in the top 1 in 100 million”, or top 1 in 10 possible genetic codes. Why would you define a subset of 100 million (or 10) and then say it’s in the top of that? It seems to me a strange way of stating it.
Is another way of saying what you intend to convey, that out of 100 million genetic codes picked at random, the genetic code is usually going to be among the best ones in that set?
Or that, on average you’re going to go through 100 million codes for every better one (than the genetic code we have) you find?
Even if one chosen at random?
Rumraket, to vjtorley:
Rumraket,
The phrase “in the top 1 in 100 million” comes from the fact that Vincent is interpreting Koonin’s statement for us.
Koonin writes that “the code is indeed highly resilient, with the probability to pick an equally robust random code being on the order of 10^−7 – 10^−8”. That translates directly into “the code is in the top 1 in 100 million” (assuming that by “equally robust”, Koonin really means “at least as robust”).
My only quibble would be to amend Vincent’s statement…
…to read:
We don’t actually know, from the information given in Koonin’s statement, how many of those codes are better.
P.S. Excellent OP, Vincent. I’ve bookmarked it for future reference.
And you actually believe this? And evolutionists actually believe this. And it gives them no pause for thought that they believe this.
That is what is so astounding.
We don’t actually know that they are equal to.
Walto is concerned that KN is using a weird worldview.
So you mean a perfect code would be one in which there are zero errors ever?
So every thing alive would be the exact same thing?
Yes, and yes, and no.
We know that this is how you feel. I fully anticipated that this woulde be your reaction. You would express amazement, but never get around to explaining what the problem actually is. You never get around to explaining why. You just declare your disbelief, that’s it.
I don’t know how to refute an incredulous stare.
I’m sorry but speak for yourself here.
We do actually know what the properties of the genetic code are, and how altering those properties would affect the code’s performance in terms of error minimization.
That might be what a perfect code is, it just isn’t relevant to what we are talking about. Nobody says the gentic code has to be perfect. But if it was designed by a super-intelligence, it would be expected to be the best possible code with respect to reducing the deleterious effects of mutations.
It doesn’t have to be perfect, to be the best possible code out of all the 10^84 possible codes.
No, why do you think that? How is that question at all relevant to the nature and performance of the genetic code?
LoL!
It’s not the best possible code therefore it wasn’t designed by a super intelligence?
Hi keiths,
I’m glad you enjoyed reading my OP. Thank you.
You are correct.
Hi Mung,
You asked: “It’s not the best possible code therefore it wasn’t designed by a super intelligence?”
If you look at my OP, you’ll see that I was very careful not to argue that. What I wrote was:
“That means that there are 10^76 possible genetic codes that are better than it. To make matters worse, it’s not even the best code in its local neighborhood. It’s not “on top of a hill,” as it were. It’s about half-way up the hill. Now ask yourself: if the genetic code were intelligently designed, is this a result that one would expect?”
I’m well aware that Intelligent Design hypotheses come in various degrees of sophistication. Rumraket is entirely correct when he writes: “Nobody says the genetic code has to be perfect. But if it was designed by a super-intelligence, it would be expected to be the best possible code with respect to reducing the deleterious effects of mutations.” That would certainly be our Intelligent Design hypothesis Mark I. And if scientists had actually discovered that it was the best possible code, then I’m sure every Intelligent Design theorist from here to Seattle would be trumpeting this result as proof that it was designed.
Now, the fact that the standard genetic code is not optimal could be explained by the fact that it was subject to additional constraints – in other words, minimizing errors isn’t everything. Fair enough. But “half-way up the hill” is an odd result that I would not have expected. What, not even a local maximum? It’s going to take a very sophisticated Intelligent Design hypothesis to explain away that one.
But if you can propose such a hypothesis, then you’re most welcome to do so.
Hi Rumraket,
Or that, on average you’re going to go through 100 million codes for every better one (than the genetic code we have) you find?
Yes, that’s a good way of putting it. Except that I should say (h/t keiths) “equal to or better.” Cheers.
Do we? Do we know why some genes are turned on and some genes are turned off? Do we know how the genes became regulated during development? Do we know how the code came to exist in the first place? I think your statement is completely untrue.
How is it not relevant? Its not relevant what “good” means as far as a code, to know if something is better and something is worse? This is starting to sound like some of your sides bizarre fitness arguments. If good means less mutations and bad means more, then the best is no mutations ever, and the worst is all mutations. So if a genetic code never had any mutations during reproduction, then of course every new organism would be exactly the same right? ONE genetic code.
Or at least ONE genetic code for every species. So that every tiger would be like every other tiger. Every fish would be exactly like every other fish, and every human would be exactly like every other human. That is what VJ is saying a designer would do, if they were intelligently designing a genetic code. But how does he know that is what a designer would likely do?
Where exactly is the justification for VJ saying that? There is none. So instead, his statement should simply be, there are billions of other possible codes, WITHOUT conjecturing that a designer would have used a different one, because he has no way of saying why another would be better. So this point by VJ completely fails. And yet you all have excepted it without question.
If VJ’s point about this fails, then his whole critique of Miller fails. But you all have bought into it so uncritically. And here, at least, VJ is present to try to defend that statement, but of course he won’t. Which makes this whole routine much more valid than VJ’s, because here we are simply seeing VJ critique something one sidedly, without getting the other side, a rebuttal from Miller. Its quite easy to make an argument in court about one’s side of an issue, if the other side isn’t there to refute what is said.
“The killer was with the victim at the time they were shot, they owned the gun, and there is a video of them shooting them, I declare.” Well, if that’s the case, it sure does sound like they should be in jail. However, if the other side is there to say, “No actually, the accused was in another city, and here is the video to show that. Furthermore the video you are talking about, showing them shooting someone is actually a cartoon, made months later, and using clay models.”
OHHH, well, that’s different then!
VJ’s whole argument rests on him giving his views, and all of you just accepting them with a nod, en, en, very interesting. Some skeptics you guys are.
If you don’t know the purpose of the code, how can you determine what is better and what is worse?
What makes you think complete uniformity is better?
vjtorley,
Because you see Vj, this is the problem I have with many of your posts. You say many things, but when you are challenged by some of your statements, you generally have very little in the way of defending those statements.
To me its somewhat cowardly, in the same way, that you criticize someone else’s writings, in a place like this, where they have no chance to respond to those criticisms.
You did the same thing at UD. When you were challenged by your views, you ran from answering those remarks.
Why? You mean it should be able to get good mutations but not bad ones?
It sounds like Omagains argument for the existence of evil and uphills, when he only wants whip cream and downhills.
Ahh, I see that you don’t actually know what the genetic code is, and you think it is your genomic sequence.
This is a very common misconception, but I can’t fault you for it because the term is constantly used incorrectly in the popular press, TV-shows and even by many science-writers and scientists themselves.
The ‘genetic code’ is about how nucleotide-sequence in your DNA is ultimately turned into amino acid sequence when proteins are synthesized in your cells. It has nothing to do with genes being switched on or off and it does not determine your genomic sequence. It has to do with translation.
The code is the set of “rules” that determine how nucleotide sequences in your DNA are turned into amino acid sequences of proteins.
The rest of what you write is based on this misunderstanding and is thus entirely off the mark here. The genetic code doesn’t reduce the chance of mutations, it reduces the magnitude of the deleterious effects of those mutations. And yes, we really do know this.
The genetic code doesn’t affect how your genome is replicated, it affects how messenger-RNA sequences are translated into proteins.
The typical human is born with somewhere between 100 and 150 mutations. If you had a different gentic code that had a greater error minimization ability, you would still be born with 100 to 150 mutations, because the genetic code doesn’t affect the rate of mutation at replication(or otherwise). It affects how likely it is that those mutations will have a deleterious effect, and in so far as it does have a deleterious effect, it affects the magnitude of the deleterious effect.
We know the “purpose” of the code. The code’s “purpose” is to serve as the set of rules that make it possible for your cells to make the proteins that are essential for you to be alive.
We can differentiate between deleterious effects and advantageous effects?
Is any effect at all a deleterious one?