ID proponents and creationists should not use the 2nd Law of Thermodynamics to support ID. Appropriate for Independence Day in the USA is my declaration of independence and disavowal of 2nd Law arguments in support of ID and creation theory. Any student of statistical mechanics and thermodynamics will likely find Granville Sewell’s argument and similar arguments not consistent with textbook understanding of these subjects, and wrong on many levels. With regrets for my dissent to my colleagues (like my colleague Granville Sewell) and friends in the ID and creationist communities, I offer this essay. I do so because to avoid saying anything would be a disservice to the ID and creationist community of which I am a part.
I’ve said it before, and I’ll say it again, I don’t think Granville Sewell 2nd law arguments are correct. An author of the founding book of ID, Mystery of Life’s Origin, agrees with me:
“Strictly speaking, the earth is an open system, and thus the Second Law of Thermodynamics cannot be used to preclude a naturalistic origin of life.”
Walter Bradley
Thermodynamics and the Origin of Life
To begin, it must be noted there are several versions of the 2nd Law. The versions are a consequence of the evolution and usage of theories of thermodynamics from classical thermodyanmics to modern statistical mechanics. Here are textbook definitions of the 2nd Law of Thermodynamics, starting with the more straight forward version, the “Clausius Postulate”
No cyclic process is possible whose sole outcome is transfer of heat from a cooler body to a hotter body
and the more modern but equivalent “Kelvin-Plank Postulate”:
No cyclic process is possible whose sole outcome is extraction of heat from a single source maintained at constant temperature and its complete conversion into mechanical work
How then can such statements be distorted into defending Intelligent Design? I argue ID does not follow from these postulates and ID proponents and creationists do not serve their cause well by making appeals to the 2nd law.
I will give illustrations first from classical thermodynamics and then from the more modern versions of statistical thermodynamics.
The notion of “entropy” was inspired by the 2nd law. In classical thermodynamics, the notion of order wasn’t even mentioned as part of the definition of entropy. I also note, some physicists dislike the usage of the term “order” to describe entropy:
Let us dispense with at least one popular myth: “Entropy is disorder” is a common enough assertion, but commonality does not make it right. Entropy is not “disorder”, although the two can be related to one another. For a good lesson on the traps and pitfalls of trying to assert what entropy is, see Insight into entropy by Daniel F. Styer, American Journal of Physics 68(12): 1090-1096 (December 2000). Styer uses liquid crystals to illustrate examples of increased entropy accompanying increased “order”, quite impossible in the entropy is disorder worldview. And also keep in mind that “order” is a subjective term, and as such it is subject to the whims of interpretation. This too mitigates against the idea that entropy and “disorder” are always the same, a fact well illustrated by Canadian physicist Doug Craigen, in his online essay “Entropy, God and Evolution”.
From classical thermodynamics, consider the heating and cooling of a brick. If you heat the brick it gains entropy, and if you let it cool it loses entropy. Thus entropy can spontaneously be reduced in local objects even if entropy in the universe is increasing.
Consider the hot brick with a heat capacity of C. The change in entropy delta-S is defined in terms of the initial hot temperature TH and the final cold temperature TM:
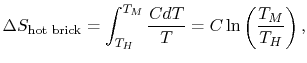
Supposing the hot temperature TH is higher than the final cold temperature TM, then Delta-s will be NEGATIVE, thus a spontaneous reduction of entropy in the hot brick results!
The following weblink shows the rather simple calculation of how a cold brick when put in contact with a hot brick, reduces spontaneously the entropy of the hot brick even though the joint entropy of the two bricks increases. See: Massachussetts Institute of Technology: Calculation of Entropy Change in Some Basic Processes
So it is true that even if universal entropy is increasing on average, local reductions of entropy spontaneously happen all the time.
Now one may argue that I have used only notions of thermal entropy, not the larger notion of entropy as defined by later advances in statistical mechanics and information theory. But even granting that, I’ve provided a counter example to claims that entropy cannot spontaneously be reduced. Any 1st semester student of thermodynamics will make the calculation I just made, and thus it ought to be obvious to him, than nature is rich with example of entropy spontaneously being reduced!
But to humor those who want a more statistical flavor to entropy rather than classical notions of entropy, I will provide examples. But first a little history. The discipline of classical thermodynamics was driven in part by the desire to understand the conversion of heat into mechanical work. Steam engines were quite the topic of interest….
Later, there was a desire to describe thermodynamics in terms of classical (Newtonian-Lagrangian-Hamiltonian) Mechanics whereby heat and entropy are merely statistical properties of large numbers of moving particles. Thus the goal was to demonstrate that thermodynamics was merely an extension of Newtonian mechanics on large sets of particles. This sort of worked when Josiah Gibbs published his landmark treatise Elementary Principles of Statistical Mechancis in 1902, but then it had to be amended in light of quantum mechanics.
The development of statistical mechanics led to the extension of entropy to include statistical properties of particles. This has possibly led to confusion over what entropy really means. Boltzmann tied the classical notions of entropy (in terms of heat and temperature) to the statistical properties of particles. This was formally stated by Plank for the first time, but the name of the equation is “Boltzmann’s entropy formula”:

where “S” is the entropy and “W” (omega) is the number of microstates (a microstate is roughly the position and momentum of a particle in classical mechanics, its meaning is more nuanced in quantum mechanics). So one can see that the notion of “entropy” has evolved in physics literature over time….
To give a flavor for why this extension of entropy is important, I’ll give an illustration of colored marbles that illustrates increase in the statistical notion of entropy even when no heat is involved (as in classical thermodynamics). Consider a box with a partition in the middle. On the left side are all blue marbles, on the right side are all red marbles. Now, in a sense one can clearly see the arrangement is highly ordered since marbles of the same color are segregated. Now suppose we remove the partition and shake the box up such that the red and blue marbles mix. The process has caused the “entropy” of the system to increase, and only with some difficulty can the original ordering be restored. Notice, we can do this little exercise with no reference to temperature and heat such as done in classical thermodynamics. It was for situations like this that the notion of entropy had to be extended to go beyond notions of heat and temperature. And in such cases, the term “thermodynamics” seems a little forced even though entropy is involved. No such problem exists if we simply generalize this to the larger notion of statistical mechanics which encompasses parts of classical thermodynamics.
The marble illustration is analogous to the mixing of different kinds of distinguishable gases (like Carbon-Dioxide and Nitrogen). The notion is similar to the marble illustration, it doesn’t involve heat, but it involves increase in entropy. Though it is not necessary to go into the exact meaning of the equation, for the sake of completeness I post it here. Notice there is no heat term “Q” for this sort of entropy increase:

where R is the gas constant, n the total number of moles and xi the mole fraction of component, and Delta-Smix is the change in entropy due to mixing.
But here is an important question, can mixed gases, unlike mixed marbles spontaneously separate into localized compartments? That is, if mixed red and blue marbles won’t spontaneously order themselves back into compartments of all blue and red (and thus reduce entropy), why should we expect gases to do the same? This would seem impossible for marbles (short of a computer or intelligent agent doing the sorting), but it is a piece of cake for nature even though there are zillions of gas particles mixed together. The solution is simple. In the case of Carbon Dioxide, if the mixed gases are brought to a temperature that is below -57 Celcius (the boiling point of Carbon Dioxide) but above -195.8 Celcius (the boiling point of Nitrogen), the Carbon Dioxide will liquefy but the Nitrogen will not, and thus the two species will spontaneously separate and order spontaneously re-emerges and entropy of the local system spontaneously reduces!
Conclusion: ID proponents and creationists should not use the 2nd Law to defend their claims. If ID-friendly dabblers in basic thermodynamics will raise such objections as I’ve raised, how much more will professionals in physics, chemistry, and information theory? If ID proponents and creationists want to argue that the 2nd Law supports their claims but have no background in these topics, I would strongly recommend further study of statistical mechanics and thermodynamics before they take sides on the issue. I think more scientific education will cast doubt on evolutionism, but I don’t think more education will make one think that 2nd Law arguments are good arguments in favor of ID and the creation hypothesis.

Petrushka’s Second Law of Intelligent Design:
All ID arguments can be reduced to ignorance and incredulity.
Prime example of ignorance:
http://www.antievolution.org/cgi-bin/ikonboard/ikonboard.cgi?act=ST;f=14;t=7305;st=5010#entry207631
Nature can’t evolve the ability to digest lignin.
Now let’s see how long it takes for the ID crowd to say this obviously couldn’t have evolved.
Interesting, that the Designer seems to have created the ability to feed on ignorance.
The current objection (Granville’s paper) I believe is such that highly specified microstates do not become statistically more likely due to the uncertainty of other microstates. I thought he advanced a good argument, but I tend to agree with OP on this.
Thanks for that link.
I wondered about termites, when the ID folk posted that argument.
I’m still puzzled as to why they posted that. It seemed such a weak criticism of evolution.
I am still puzzled that Granville Sewell has been making such an obviously wrong argument. Given that he is a mathematician, I am puzzled that he does not see that it is obviously wrong.
Actually the original article mentions termites, but they argue that nothing obtains energy from metabolizing lignin. But if bacteria release CO2, they are “burning” it.
The original article answers its own question. Digesting lignin is energy inefficient. Organisms break it down to get to the cheap and plentiful fuel. There’s no profit in trying to use lignin as fuel.
Sounds a bit like citrate to me. Maybe there’s a project for some up and coming Lenski.
Sewell’s arguments about the 2nd law of thermodynamics have been discussed at length in five threads on this blog. See Category 2nd Law of Thermodynamics.
It boils down to Sewell’s poor understanding of thermodynamics and (especially) statistical physics. His technical argument, based on the concept of “X-entropy,” is a good illustration of that. As I explained here, Sewell’s specific example of X-entropy is a botched version of configurational entropy. Needless to say, configurational entropy is subject to compensation. So Sewell fails on technical merit as well as on the big picture.
But none of this matters. Sewell himself does not care all that much about his technical arguments. He falls back on the good old argument from incredulity. Here is his latest response to Sal (conveyed by Denyse):
Sewell concedes that life does not violate any actual formulations of the 2nd law. Then he says that it contradicts some dumbed-down versions. Oh well, garbage in, garbage out.
Sewell ends his rejoinder thus:
We feel your pain, Granville, and as a gesture of good will we linked your article in this quote.
You have outgrown the UD sandbox. Come out to play with big boys.
Meanwhile, say hi to all those good physicists.
P.S. Sewell posts his own response thread at UD. Wow! 4 threads on 2 forums in an hour.
The misrepresentations and misconceptions about thermodynamics and statistical mechanics are just a small sample of why ID/creationists never get the science right in any area of science. To get it right means that it will never comport with sectarian dogma; ID/creationists have to get it wrong. Henry Morris – and probably A.E. Wilder-Smith – started them on this track
Confusing disorder with entropy is just the tip of the iceberg; even as the comparison of the mixing of different colored marbles with entropy still gets it wrong.
And the system of the mixture of carbon dioxide and nitrogen is not an example of entropy decreasing. In order to separate those two gases, they have to be brought into contact with a colder system in order to dump their kinetic energy. That energy flows out of the carbon dioxide and nitrogen system into a colder environment meaning that entropy increases overall.
It’s the same with any form of condensation; energy must be released. If one confines one’s attention to only the condensing system, one loses the big picture. The entropy of the condensing system can decrease only because energy is transported out of the system to the surrounding environment or to other systems. Again, the overall entropy increases.
Marbles do not interact with each other as atoms and molecules do. If one could somehow color all the molecules of a gas in one half of an isolated container a different color than the identical molecules in the other half of that same container, one would not find the entropy increasing as they continue to mix and interact. (by the way, this is a thought experiment; it is not possible to “color” anything without changing or adding to the molecules already there)
In the case of the gas molecules, color has nothing to do with entropy if the molecules are identical. The same would be the case for the marbles. Marbles don’t spontaneously mix like gas molecules (there is a very importan reason why); but if they did and were otherwise identical, the entropy would not increase.
There is no difference between shaking a box of identical molecules of the same color and shaking a box of identical marbles of different color. Entropy has nothing to do with disorder.
As already mentioned, ID/creationist misconceptions about biology and evolution don’t stop at just biology and evolution. They are every bit as bad in every other area of chemistry and physics as well. If ID/creationists actually understood just how badly they misrepresent and misunderstand science, they would be too embarrassed to try to foist ID/creationism onto other people’s children; and that is the basic problem. There would be no ID/creationism if its pushers would sit down and actually learn the science.
It’s simple, Neil. Physics is not just a lot of hard math thrown together. It is much more than that. Sewell is an OK mathematician, but he is no physicist.
Never mind, Mike your prejudice against me is obvious. You can’t bring yourself to agree with me even when I criticize an ID proponent. So much so you can’t even allow the most charitable reading of what I wrote.
<blockquote>
In order to separate those two gases, they have to be brought into contact with a colder system in order to dump their kinetic energy.
</blockquote>
A mechanism of cooling was implicit when I specified a temperature range that would liquefy gaseous Carbon Dioxide. So that was already in what I wrote if you were willing to see it! Your response was deeply uncharitable, but in any case, thanks for your response.
<blockquote>
If one could somehow color all the molecules of a gas in one half of an isolated container a different color than the identical molecules in the other half of that same container, one would not find the entropy increasing as they continue to mix and interact.
</blockquote>
What, you’re unfamiliar with the entropy of mixing? No need to color gas molecules of different species since they are distinguishable. And if one wants to be anal, they do have different spectroscopic characteristics so they will be different colors if one had the right frequency of light and instruments sensitive enough. 🙂
I’d like to thank Elizabeth Liddle for her hospitaltiy. I hope this post is consistent with some of her wishes for being skeptical about our own positions.
Salvador,
Let me begin by recognizing your effort to address a serious problem with a prominent ID proponent. As a wise, albeit fictional, man said, “It takes a great deal of bravery to stand up to our enemies, but just as much to stand up to our friends.”
Granville Sewell’s misconceptions of the second law of thermodynamics have been discussed on at least three threads on this blog alone:
In every one of those threads are a number of comments that individually destroy his argument. One simple demonstration of how utterly unsupportable his position is comes from olegt’s dimensional analyis here, here, and here.
An excellent summary of the second law is provided by Mike Elzinga here and here. His concept test should be required of anyone participating in the discussion. I suspect that Sewell cannot pass it.
Given all of this existing information, I would like to ask you a favor. Would you be willing to post these links at Uncommon Descent and ask Granville Sewell to reply in either venue? I would do so myself, but UD is not particularly open to dissenting views. Perhaps we can reach some sort of consensus on this one issue within the larger debate.
Never mind, Mike your prejudice against me is obvious. You can’t bring yourself to agree with me even when I criticize an ID proponent.
Interesting. I saw his post as extending yours rather than disagreeing. But speaking of charity. your post ignores the existence ot three entire threads devoted to the topic. What are we to make of that?
You can take it personally if you wish, I don’t care; but ID/creationists have been playing games with entropy since the early 1970s. When they get called out on it, the best they ever do is suggest to their followers that it is probably not a good idea to use “the argument from the second law of thermodynamics.” But then, guess what, they turn right around and use it again in every new venue or with people who are not likely to have heard the “argument” before.
This has been going on for over 40 years now; I’ve been around all that time watching it. You can go to ICR website right at this very moment and type in “entropy” or “thermodynamics” or “second law” into their search box and pull up everything Morris and Gish harassed teachers with. They never retracted any of it.
So what do you think is going on? Why didn’t Sewell submit his “paper” to Physical Review Letters?
Why don’t you try to explain what the color of marbles has to do with the number and distributions of energy micostates? In fact, why don’t you try to explain what playing with marbles has to do with how atoms and molecules interact?
Even though it has been repeatedly pointed out to them by physicists for over 40 years, it seems that every time an ID/creationist brings up entropy and the second law, they never learn that spatial disorder has nothing to do with entropy. The entropy of mixing has nothing to do with disorder; it has to do with the redistribution of energy microstates. You can learn this just by picking up any of the good textbooks on thermodynamics and statistical mechanics.
And it doesn’t do any good to start quoting “authorities.” Physicists have long been familiar with the misconceptions and faux pas analogies by well-meaning textbook authors and writers of popularizations. Anyone can look at all of the different methods for computing entropy and learn that order has nothing to do with it. You won’t find order in k ∑ pi ln pi or in ∫ dQ/T. You won’t find it in k ln Ω.
This “retraction” game with the second law is old news. It will be trotted out once again, just as Sewell did. It’s a taunt to get publicity and a free ride on the coattails of someone in science. It all goes into the political game of maintaining a façade of “legitimacy.” It’s not about science.
But you already know that.
Why don’t you try to explain what the color of marbles has to do with the number and distributions of energy micostates?
Microstates aren’t all about energy but also position, that’s why there is MIXING entropy. See the Gibbs Paradox.
Given all of this existing information, I would like to ask you a favor. Would you be willing to post these links at Uncommon Descent and ask Granville Sewell to reply in either venue? I would do so myself, but UD is not particularly open to dissenting views. Perhaps we can reach some sort of consensus on this one issue within the larger debate.
I posted a link to your comment on the basis of the hospitality Elizabeth extended to me here. Your comment provides the necessary links. See my updated post at UD.
Thanks for your kind words. This is not a happy day for me….
<blockquote>
But speaking of charity. your post ignores the existence ot three entire threads devoted to the topic. What are we to make of that?
</blockquote>
That I don’t visit skeptical zone much except to post, not read. 🙂
I posted in my own words for the sake of my side, not the evolutionist side. It might not persuade my side to read the comments of hostile evolutionists like Mike Elzinga, but they might give me an audience….
You need to take that concept test to which Patrick linked above. The answers are also posted somewhere if you are really interested. And I would suggest that you learn about Gibbs Paradox instead of attempting to taunt me with it. I have been immersed this stuff most of my life in research. I actually use these concepts in getting things done.
It is common on for ID/creationists to take offense at bluntness. They use it as a ploy to get sympathy. Sympathy doesn’t nudge them to learn; it is, instead, an attempt to get the scientists they taunt to treat them as equals and as professionals. That is not how one gets respect in science.
And if you think I’m “hostile,” just watch what happens to you if you ever start to understand how science is being distorted by ID/creationists and you start trying to correct them from your “sympathetic” position of “understanding of where they are coming from.” I would suggest that you may encounter some real hostility born of threats to their desire for celebrity and authority.
Many of us here have been all through this with ID/creationists for over four decades now. This is all old stuff you are rehashing here.
Get some guts and take it to your “cohorts” with some honesty. Then you’ll find out.
” Perhaps we can reach some sort of consensus on this one issue within the larger debate.”
Dr. Sewell has already reached consensus in the face of what I wrote. He said:
“Obviously the origin and evolution of life do not violate the second law as stated in the early formulations you quote ”
And what formulations did I quote? Clausius and Kelvin Planck which according to Wiki are two of the 3 most prominent formulations. I did not go into the formulation by Constantin Carathéodory but if it is equivalent to the other two, then evolution does not violate all the most prominent formulations of the 2nd law, only maybe some obscure ones that are not standard, if that. So, imho, a major consensus has been reached….
If a consensus has been reached, one wonders why 2LOT keeps coming up. Over and over.
Is it wishful thinking? The desire to find some lawful physical barrier to evolution?
One might just as well try to find a barrier to photosynthesis.
It appears on the surface that Sal has some vague awareness of the problems with using the second law against evolution. However it doesn’t appear that he has grasped all the ramifications of that awareness yet. There is very much more, even after leaving out all the accumulated misconceptions that are rampant in the ID/creationist community.
If there is a “consensus”, it doesn’t explain why Sewell appears to be upset and doesn’t want to discuss it any further because he claims he has been discussing it for 11 years. It doesn’t take 11 years to learn about thermodynamics and statistical mechanics; especially if one already has the math skills in hand.
So what has Sewell been doing and why? I suspect it’s not over and the word games about entropy will continue.
Maybe some progress; but we shall see. I already see some “problems” being conceptualized over on UD. Clearly they don’t get it yet. After 40+ years a sudden flash of insight? The next round will likely be something about “organization.” That appears to be ripe for misconceptions and word gaming. More to come I’m guessing. Evolution just can’t be allowed.
And the origins of life? Oh my!
Granville Sewell now says over at UD:
While one may quibble with this statement (for example, is decrease of entropy “increase in order”, does one import an “amount of entropy”, and what about those X-entropies), Sewell has a point here. But that point has long been appreciated by all critics of the creationist 2LOT argument. For energy to concentrate in life, yes, it does have to flow into life across a boundary, just as Sewell says. So does it?
Where could it possibly come from? Well, Dr. Sewell lives in El Paso, Texas. It gets awfully hot down there. I hope he is careful to use sun block. The sun. That’s where most of the energy for life comes from.
The mystery about Sewell’s argument is that when he comes to apply it to evolution, he applies it in ways that make no sense. He makes it sound as if energy concentrates in life for no reason. But we all learned in middle school science where that energy comes from.
If his argument were that energy does not flow into life from the sun, then how does he account for the growth of plants? I have made several posts at Panda’s Thumb pointing out that Sewell’s argument cannot explain how plants can grow. (I’m hardly the first critic to make points like that). He seems to have no comment on this. Even if he switches to arguments about X-entropies and talks about concentrations of carbon, doesn’t that too make the prediction that plants can’t grow? Because as far as I can see that is a prediction implied by all of his arguments.
and yet Mike wrote
and mixing entropy deals with the position, and even from this wiki entry on microstates
http://en.wikipedia.org/wiki/Entropy_(statistical_thermodynamics)
So microstates involve position, position, position! And position was of interest to Gibbs when calculating mixing entropies.
From
http://en.wikipedia.org/wiki/Gibbs_paradox
Hmm, I mentioned a box with a partition separating marbles distinguishable by color, and I also mentioned it was an illustration, and then followed it with a discussion of gases. You couldn’t put the ideas together obviously….the marbles analogy was meant to help illustrate the mixing entropy associated with gases. This entropy would not mean much if one didn’t care about the relative position of molecules would it? 🙂
and yet Mike wrote
Because the issue with mixing has to do with position aspect of a microstate of a particle. If you didn’t care about the position of particles, how can you tell that they were mixed or un mixed. The colored marbles help illustrate the increase in entropy due to mixing.
Formally speaking, when the partition is removed separating different colored marbles, more microstates are available to the marbles since there are more positions the marbles can occupy once the partition is removed. The analogy to gas atoms ought to be rather transparent, but maybe your prejudice to creationists prevented you from seeing where this was leading. You couldn’t possibly admit I might be right about something even when I’m actually criticizing an ID argument.
The entropy of mixing is described here:
http://en.wikipedia.org/wiki/Entropy_of_mixing
Just because you can find systems in which particle positions are folded into the space of microstates does not mean that entropy is about disorder. Those kinds of systems are in the minority of thermodynamic systems for which entropy is easily computed.
Instead of playing word games and quote-mining “authorities” on things you don’t seem to comprehend very well, take the concept test and explain the results.
All you need are the formulas for computing entropy and temperature. If you don’t know the formulas, check Patrick’s other links. All you have to do is plug-and-chug and then explain what you got and why it has anything to do with order.
By the way; you are not being attacked. You just don’t like people who have seen 40+ years of ID/creationist shenanigans and speaks in blunt language. Get over it and learn. Then demonstrate you understand.
“All you need are the formulas for computing entropy and temperature. If you don’t know the formulas, check Patrick’s other links. All you have to do is plug-and-chug and then explain what you got and why it has anything to do with order.”
I’ll try, but I won’t swear by my answers as I want to demonstrate that I’m willing to be corrected for my misunderstanings. I wasn’t sure I got all of Patrick’s formulas, and I will be assuming equiprobable distributions.
The following is an elementary concept test.
Take a simple system made up of 16 identical atoms, each of which has a non-degenerate ground state and a single accessible excited state.
Start with all of the atoms in the ground state.
1. What is the entropy when all atoms are in the ground state?
My answer: since all are known and no uncertainty of the energy distribution, the entropy must be zero and the temperature is zero since there is no energy.
2. Add just enough energy to put 4 atoms in the excited state. What is the entropy now?
I’m going to have trouble with this one. Persuming that we must maintain 4 atoms in the excited state at all times, if my counting is correct, the number of states consistent with this is:
16 * 15 * 14 * 13
Since there are 16 possible was to occupy the first excited atom, and given that the first excited atom is fixed, there will be only 15 for the 2nd, 14 for the third, 13 for the 4th
Thus the entropy S = k ln (16*15*14*13)
There is probably a more elegant way of expressing this with factorials and combinations or permutations. I’m making the implcity assumption even though the atoms are identical, in principle they are distinguishable.
3. Add more energy so that 8 atoms are in the excited state. What is the entropy now?
Similarly S = k ln (16 * 15 * 14 * 13 * 12 * 11 * 10 * 9)
4. Add still more energy so that 12 atoms are in the excited state. What is the entropy now?
Similar to 2 but the reverse where we count the number of atoms in the ground state since so many are in the excited state
S = k ln (16 * 15 * 14 * 13)
5. Add more energy so that all atoms are in the excited state. What is the entropy now?
entropy is zero but temperature is higher, this is not a violation of the 3rd law as it is possible that atoms can be in the non-ground state but 100% determined to be in a particular state at a non zero temperature. This can happen with super fluids and Bose-Einstein condensates. This may be as some variance with classical thermodynamics. I don’t know.
6. Rank order the temperatures in each of the above cases.
Temperature is ranked from the ones with fewest in ground state to those with the most — exactly the order you laid out.
I look forward to your corrections.
Actually, there is more than a technical quibble here. Sewell’s description of compensation is misleading.
As I explained here (on a technical level) and here (non-technical summary) he claims that entropy of a system tends to increase unless it is exported through the boundary. This statement is seriously wrong because it isn’t entropy that is imported or exported, but rather energy or particles.
Sal, you can see that your entropy calculation cannot be entirely correct because it is self-contradictory.
In Point 4, you give the entropy of ln(16×15×14×13) by looking at excited states. If you look at the ground states you get a different number, ln(16×15×…×6×5). The two answers are inconsistent with each other.
The error is in miscalculating the number of microstates. For example, with two excited atoms the number of microstates is not 16×15. While it is true that you can pick the first excited atom in 16 different ways and then another one in 15 ways, you count every combination twice. For instance, you can first pick atom 1 and then atom 5 or first atom 5 and then atom 1. This, however, yields the same microstate. So you have to divide by 2 to compensate for the double counting.
With 4 excited states, the number of microstates is 16×15×14×13/4! = 16!/(12!×4!). This last result is symmetric in the number of ground and excited states, so if you compute it the other way around, by counting excited states, you get 16!/(4!×12!), i.e., the same result.
olegt,
thank you. It appears I used the permuation formula
P(n,k) = n!/ (n-k)!
instead of the combination forumula
C(n,k) = p(n,k)/k! = n!/[(n-k)!k!]
In Point 4, you give the entropy of ln(16×15×14×13) by looking at excited states. If you look at the ground states you get a different number, ln(16×15×…×6×5). The two answers are inconsistent with each other
….
For instance, you can first pick atom 1 and then atom 5 or first atom 5 and then atom 1. This, however, yields the same microstate. So you have to divide by 2 to compensate for the double counting.
Thank you, that clarifies my mistake.
You are making progress. 🙂
While you are working on this concept test, here are some notions to float in the back of your mind as you begin to explore the meaning of entropy.
Not everything in the universe is a gas or a liquid. So how do you think entropy relates to solids – crystals or polycrystals, for example? The atoms are locked in position and their mean position doesn’t change over wide temperature ranges. So where are the microstates and how is entropy calculated for something like a polycrystalline solid (Hint: it is NOT about positions)?
What do your calculations with the two-state system do with spatial positions of the atoms? Does it make a difference? Suppose those two-state atoms are locked in a matrix of other atoms that do not participate in the energy exchanges? How are they ordered – or not ordered?
Do you know what the definition of temperature is? Do you know how it relates to entropy and energy?
You are making progress.
Thanks!
So how do you think entropy relates to solids – crystals or polycrystals, for example? The atoms are locked in position and their mean position doesn’t change over wide temperature ranges. So where are the microstates and how is entropy calculated for something like a polycrystalline solid (Hint: it is NOT about positions)?
The energy is in the vibrations, and the energy in the vibrations can occupy various levels. I would expect there would be in many cases where the vibrations can happen such that there are only discrete energy levels associated with a vibration (analogous to a particle in a box or vibration modes of a string). In higher temperatures, there are more ways that the energy can be distributed since more microstates are accessible given the higher energy levels from the vibrations. This relates to thermal entropy.
What do your calculations with the two-state system do with spatial positions of the atoms?
Nothing except to assume there is not energy exchange between the paritcles.
Does it make a difference?
Not for calculating thermal entropy. As pointed out with the Gibbs paradox the other forms of entropy (like mixing) are in the eyes of the beholder.
Suppose those two-state atoms are locked in a matrix of other atoms that do not participate in the energy exchanges? How are they ordered – or not ordered?
I don’t like using the word ordered for thermal entropy. It seems deeply inappropriate for the description of thermal entropy and for the 16-atom case you described.
For specific configurations or where position matters (such as in mixing entropy) reference to might make some sense, but I don’t use the second law Clausius formulations in such cases.
Do you know what the definition of temperature is?
A crude definition is mean kinetic energy. I seem to recall someone relating temperature to widness of the maxwell speed distribution in plasmas. Something I didn’t quite understand is directionally dependent temperatures in plasmas.
Do you know how it relates to entropy and energy?
Temeprature is a measure of the mean kinetic energy which is the macro state of the system. The higher the temperature the larger number of ways that various microstates can realize that temperature state. This larger number of microstates corresponds to increases in thermal entropy. Thus heating creates higher thermal entropy and cooling creates lower thermal entropy. This may or may not have much relevance to mixing entropy or other forms of entropy considered in statistical physics.
That is my best understanding. I welcome you comments and corrections. Thank you.
There is an example of the Einstein oscillator model in one of Patrick’s links.
If that is the case, how do isolated systems ever come to equilibrium? That would mean that you can’t use the number of combinations to count microstates because the system would be in only one state (zero entropy) and you could not make the assumption that those other states are available. (Big hint: matter interacts strongly with matter. It is extremely difficult to make an isolated system let alone one in which all of its constituents are also isolated from each other.)
Nature doesn’t care about the “eyes of the beholder.” Don’t get hung up on the Gibbs paradox before you understand how to count microstates. Then the resolution of the Gibbs paradox becomes obvious.
Mean kinetic energy per degree of freedom.
The formula is in the links Patrick provided. You should also look at all of his links. You will need that formula to calculate the temperatures of the two-state system as well as for any other system in which you enumerate energy microstates. It’s an important definition; you need to comprehend its implications.
Another point that Patrick pointed out in his links: What does a dimensional analysis of Sewell’s carbon X-entropy tell you about the appropriateness of its use in his calculation? Have you ever been taught how to check units when doing calculations?
I accept that the issue of “importing entropy” is more than a quibble. The point I should have made more clearly is that you can reformulate Sewell’s argument in terms of importing energy, and then it is OK. It is just that he then takes this uncontroversial argument and is blatantly misapplying it if he argues that it is some kind of refutation of evolution.
I do note that Sewell now says that arguments in terms of what he calls “thermal entropy” do not pose any problem for evolution. But he seems to think that there are “other formulations” of entropy that do pose a problem for it. Perhaps the X-entropies that you and others here have done such a good job of debunking.
I am just wanting to make the point that even those other arguments would make it impossible for plants to grow.
Joe— even that reformulation is incorrect. Energy does not have to be imported for the entropy of a system to decrease. For example, when energy leaves the system, like when the entropy of a cup of hot coffee decreases as it cools on my desk (transferring heat from the system to the surroundings). In fact, the energy of the system can even remain constant and the entropy decrease (like during the isothermal compression of an ideal gas).
Be that as it may (and is), the explanation of how life comes to have such a concentration of energy in it is that the energy comes flowing in, mostly from the sun. Without that inflow both evolution and plant growth would have problems.
That’s right, for the special case of life on our earth (actually, for the special case of photosynthesis — probably not true of all other terrestrial metabolism which is primarily exothermic (in the thermodynamic sense)). But Sewell’s claimed tautology is neither a tautology nor is it true.
Energy sources – such as the Sun or hydrothermal vents or nuclear radiation – perform several functions with living organisms, and very likely in abiogenesis.
For organisms to be kept in a soft enough state to be viable life forms, they have to be maintained within a rather narrow temperature window. Life forms that are frozen solid or in a liquid or gaseous state don’t work very well.
There are usually higher energy photons that “tickle” chemical processes such a photosynthesis or chemosynthesis by being above the general background level of energies, but not in such quantity so as to destroy the system.
Nearly all complex systems that don’t appear to be viable because of their complexity have to have been created in an energy cascade, with the products being shuttled into more benign environments before they are torn apart again and where they can survive. “Pumping” systems allows access to energy states that cannot be reached in a “warm little pond.” Catalysis is also another way of pulling down barriers to produce products that would otherwise not be produced in a constant background environment.
We look for life forms, as we know them, in environments that are in the range of energies in which water is a liquid. But there is no reason in principle that other soft matter systems could not exist in some different liquid at other temperatures and be maintained in a soft-matter state that could lead to the complexities we see in the life forms here on Earth.
This is one of the most interesting things that come from a proper understanding of the thermodynamics of chemical systems. The possibilities are nearly endless; and that is one of the reasons that the search for the recipe that led to life here on Earth is so difficult. There are large swathes of energy ranges and mixtures of chemicals that have to be explored and eliminated.
Worst of all, we could easily overlook something right under our noses (or even in or noses) because our search tactics destroy what we are looking for. It is extremely difficult to plan for every possibility; there are just too many. It requires lots of clever searching, lots of guesswork, and lots of just plain luck. That is why the research is done on so many fronts.
Without a proper understanding of chemistry and thermodynamics and the second law, the search would have no guidance whatsoever. It would be basically a random hunt. And given the complexities of what we are searching for, that knowledge is crucial.
Salvador,
I see that Rob Sheldon has joined the discussion with a new post at UD. A quick perusal suggests to me that he does not recognize the flaws with Granville Sewell’s position that have been explained here in great detail. He also seems to be ignoring the fact that the statistical mechanics derivation of entropy is based on energy microstates, not configuration. Further, he seriously muddies the waters by bringing up Shannon information, measurement of entropy in cells, and live vs. dead cows.
If you happen to participate on that thread, please do invite him to join us here. I’m interested in going deeper into his argument.
My mistake, yes of course, thank you.
But the Gibbs paradox show the significance of how we perceive things. From the link, “This insight suggests that the idea of thermodynamic state and entropy are somewhat subjective.” But you are right I need to learn more about counting microstates.
Does this relate to temperature being the partial derivate of energy with respect to entropy with number and volume held constant (in which case, since number and volume are constant, the partial derivatives can become exact derivatives)? Thanks in advance….
where:
S(N,V,E) = k ln W(N,V,E)
Yes, but more practice is always helpful. The statement of Temperature is the “mean kinetic energy per degree of freedom” vs. “mean kinetic energy” makes more sense. Since temperature uses scales like the Kelvin scale, wherease energy is in Joules, I was a litte fuzzy on how the dimensions would be lined up. Correct me if I’m mistaken, but since entropy is measured in Joules/Kelvin, and if temperature is:
T = (partial E/ partial S) ; N,V constant
then this will result in Temperature being in units of Kelvins if one uses the Kelvin scale.
I haven’t visited those links yet.
I may have missed the appropriate discussion, I will look again when I have time. Thanks again for the information and corrections.
The post was not put up by Rob Sheldon but by “News”. There is no link to his original statement. But as I pointed out, the key concession has been already stated, and there is no need to pile on. What Granville already said:
You wrote:
I’m already in enough hot water as it is. I’m not in a position to ask many of my colleagues for favors. That said, I’m expendable, so I feel freer to speak my conscience.
There are those in the ID community who are glad I broke ranks and articulated the objections they privately held. For that reason, I haven’t been axed nor received any more retribution than what is on display at UD. For the time being, I’ve had to be the voice of the minority of dissent that has been silent up until now. So in the interest of me not burning any more bridges with my colleagues, I wish to avoid getting more of them involved here at Skeptical Zone on this topic. Perhaps other topics would be good. I find the discussion with first rate critics to be fruitful.
I do not have a professional reputation to defend. I’m in the financial industry, I’m not a scientist. However, Granville is a professor with a reputation to uphold. The stakes are higher for his career, and I’m sorry to see this episode transpire for his sake. I have wondered why no one stepped in the ID community and pulled him aside to say, “hey wait a minute, how does that line up with Clausius? You can’t just pick and choose the formulation that gives you the result you want, you ought to be able to prove what you want from ALL formulations of the 2nd law.”
He is a published ID author and has been editor of an anthology that included other ID authors. My involvement in this matter will probably damage relations with many on my side, but I felt the truth was on my side. Granville made the point now more explicit which I wished he made years ago:
If I didn’t stand up, I’d have colleagues trying to defend ID based on Clausius and Kelvin-Planck, and they will suffer the predictable consequences of defending such erroneous notions. I couldn’t sit on the sidelines and let that happen.
Each time I hear an ID proponent try to invoke the 2nd law, I’ll have something to point out:
That is what I hoped he would say for his sake and everyone’s sake. Obviously, I’m not as knowledgeable in physics as those here on this thread, but I knew just barely enough to come forward and make the critique I made. I’m glad with much difficulty Granville and I can agree on some points.
Actually what you did was to stir up the nest over there at UD so that those of us who have some experience profiling misconceptions and misrepresentations can see what Sewell’s admirers are “thinking.” That might be useful; but from what I have seen so far, it’s pretty much the same old stuff with some hint of new attempts to word-game entropy and the second law.
The “controversies” they imagine about the second law and entropy – along with the application of these to complex systems – exist in their own minds. This kind of defensive narrative is the result people having little understanding of fundamental concepts but with a strong ideological drive to bend concepts to fit their ideology. It’s bad science no matter how it is word-gamed.
That pattern has been going on for over four decades now. There is little hope that they will actually make an attempt to learn the concepts. And there is no need to speculate about Sewell’s relationships with physicists; his own misconceptions speak for themselves. How that plays out in his professional life is his problem.
Let’s review what Sewell wrote:
If one stops here then there is an impression that Sewell agrees with you. It turns out, however, that that was just an introductory sentence, not a conclusion but a prelude to an argument.
Uh-oh, I see a but! Granville agrees with you only in part and might disagree in whole. Let’s keep reading.
So much for an agreement! Sewell finds an introductory physics textbook for non-physics majors and in that book eyes some dumbed-down versions of the second law (version 1 is suitable for high-school students, version 2 for kindergarten). He then thinks that such nonsense is worth publishing in a scientific journal! And just in case, he wants more examples of mangled physics, so he turns to pop-sci literature:
You can quote-mine Sewell all you want, but you can’t save him from himself. Every time he opens his mouth he says darnedest things.
For anyone interested in the thought processes and discussions that lead to the construction of misconceptions and misrepresentations, that “discussion” going on over at UD is quite instructive. It’s as though one has a front-row seat looking in on a cargo cult’s innermost deliberations on how to get the airplane to come down to visit them.
A better definition of temperature is the inverse of the rate of change of entropy with respect to energy:
1/T = dS/dQ
Temperature as mean kinetic energy per degree of freedom (which is what is usually taught in introductory thermo courses) is only valid for ideal gases. It’s a decent heuristic more generally, but there are some less common situations where Boltzmann law beaks down badly, such as in Mike’s concept test. Thermodynamic temperature can be negative and even infinite (or both).
Salvador,
I find this excerpt fascinating. In what sense are you “expendable”? The terms you are using and the view you are providing into the ID culture are much more redolent of a political or religious movement than of a scientific one.
Modulo a few relatively minor details, you are correct about the second law of thermodynamics argument and Granville Sewell is demonstrably wrong. This isn’t a subjective matter of opinion. Yet, it appears you are worried about being ostracized when, if the ID movement valued knowledge and truth, you would be lauded.
If you can’t change the culture you’ve chosen to immerse yourself in, perhaps you should consider changing the culture you’ve chosen to immerse yourself in.
I’ve stated before that I do not argue that ID is science. I am not a scientist, hence my relationship with the ID community is one of a person with shared interest, even if based on a weakly believable hypothesis. So what is happening is that I am straining my friendship relationships, not my working ones, since blogs are not professional working science, they are discussion groups. I’m expendable from the discussion group and club and group of friends.
See: http://theskepticalzone.com/wp/?p=719
Serious science is what Olegt does. Blog communities aren’t science.
I’ve been ostracized by some (but not harshly) and others in the ID movement have lauded me in private for what I said.
I’m worried about hurting those I care about. If it were my business to correct every wrong idea my friends had, I’d have no friends.
Hey, I’m here with you guys. Disagreeing most of the time, but on occasion agreeing and even retracting what I’ve asserted on occasion.
Scientific cultures aren’t without political and religious fights either. It is widely believed that the harsh political battles in the scientific community contributed to Boltzmann’s suicide. So even if I joined the evolutionary community, one should not presume that truth is necessarily the foremost motivator.
I personally interacted with Origin of Life researcher Robert Hazen. He has accounts of the bullying he suffered at the hands of other OOL researchers like Stanley Miller. This sort of behavior is hardly consistent with being dispassionate. In fact, one would expect good science to be passionately and not dispassionately pursued.
Linus Pauling once said: “there are no such things as quasi crystals, only quasi scientists”. How are such statements scientific, they are political and not dispassionate. Further, Pauling was wrong, there are quasi crystals.
I respect the scientific community, but lets not pretend they are somehow immune from human nature.
And for what it’s worth, I do not think the evolutionary community is being held to the same high standards of science that other disciplines like Chemistry and Physics and Engineering are. I could make the criticism that philosophical agendas are in play since evolutionary biology is being let off the hook from serious critical analysis.
You might argue that ID is not held to scientific stadards either. But I don’t claim ID is science, so that criticism doesn’t apply, imho.
Finally, even supposing the ID community are all but scoundrels without education, it does not erase the fact evolutionism has serious credebility issues and that they are making claims that deserve to be investigated and challenged on scientific grounds.
“In science’s pecking order, evolutionary biology lurks somewhere near the bottom, far closer to prhenology than to physics.” — Jerry Coyne
I suspect you are being disingenuous.
As someone who has observed the ID/creationist community for over 40 years, I would suggest to you that we already know that these supposed “issues” in science – especially about evolution – have been fabricated by the ID/creationist community for socio/political purposes. Many of us have witnessed the taunting tactics of Henry Morris and Duane Gish and others at the ICR back in the 1970s and 80s. I have known biology teachers who were harassed by Duane Gish when he would show up uninvited in their classrooms.
You are familiar with the Wedge Document, and you know that document was meant for internal use only. It reveals only the tip of the iceberg of sectarian antipathy toward science and secular society.
And you know that anyone can go over to the videos at AiG and look at the demonizing of science and secular society going on over there. Ken Ham and his organization aren’t fans of science. Ham is on a crusade against secular society and other churches; and he uses a façade of science talk to sell his message.
You are certainly aware of where Henry Morris’s protégé, Thomas Kindell learned his snarky presentation tactics. Few people are fooled by the political talking points of the ID/creationist movement these days.
You know very well what that movement is and what it wants. Lee Atwater and his protégé, Karl Rove, learned a lot about how to exploit fear and loathing by immersing themselves in these kinds of community. Such tactics have become part of the working political script of the Far Right in our society.
These thermodynamics “arguments” are the unspoken foundation of all ID/creationist misconceptions and misrepresentations of evolution. They permeate the “theories” and writings of all ID/creationist leaders.
It’s all about politics; not science. And we are pretty sure you already know all that.
The US Constitution allows sectarians to worship in the churches of their choice. They can even use pseudoscience as the pillars of their sectarian dogma if they wish.
So ask yourself; why would these sectarians – who are fed and protected by a secular society – want to use the institutions of secular government to foist their sectarian dogma onto other people’s children? Does that make any sense to you?
it does not erase the fact evolutionism has serious credebility issues and that they are making claims that deserve to be investigated and challenged on scientific grounds
As far as I can tell, the only claim that ID proponents really care about is common descent, and in particular, descent of man.
Otherwise, many, if not most, ID proponents accept microevolution up to and including the common descent of organisms at the Family level.
Whether mutations are guided or unguided is an issue for theology. I’m not aware of very many people who seriously think that specific instances of intervention can be detected. I don’t know anyone who is willing to cite a specific example.
But I will repeat my statement that the history of intervention hypotheses is littered with abandoned conjectures. They fall by the wayside as knowledge advances.
stcordova,
There are *two* theories of evolution, one as described by evolutionists and one as described by IDists.
The gap between the two is enormous.
As an example:
evo: I am 6 feet tall.
IDist: There is no way you weigh 1800 pounds and I can prove it.
That’s the range of difference between ID’s strawman version of evolution and the actual theory as explained by evolutionists.
Evos claim “information” changes maybe 1 or 2 bits per generation while kairosfocus argues there is no way it can change 500 bits at a time.
Salvador,
I haven’t followed your oeuvre too closely, I’m afraid. If ID isn’t science, what do you see it as? Why do ID proponents like Dembski, Meyers, Behe, and most everyone at UD portray the claims of ID as scientific?
I suspect that the reaction you would get from telling those people that ID isn’t science would rapidly dwarf the response you are getting for disagreeing with Sewell.
If ID isn’t science, what do you see it as? Why do ID proponents like Dembski, Meyers, Behe, and most everyone at UD portray the claims of ID as scientific?
My wording was a little more nuanced. I do not claim ID is science. It may or may not be, but that debate is not relevant to whether Origin of Life via mindless causes is real. If mindless OOL is false, then the debate over whether ID is science pales in the scheme of things.
For me, ID is an inference that draws from science much like forensics. Not all inferences that are true are necessarily scientific in the formal sense. There is a coin on my counter that is showing tails. In short order it will be removed from the counter. I claim the quarter is showing tails at 19:45 7/6/2012, but this claim will be unverfiable by future experiments in not too long. Does it make the claim untrue since it is inaccessible to experiment? So, not all true statements about the physical universe are necessarily accessible to science in a direct way. Many times, all we have are weak inferences, if that….ID falls in that category.
As far as the names you mentioned, it is fair to say, some of of the byproducts of trying to support ID are in the domain of sciences such as trying to determine the feasibility that certain chemical soups will create the Turing machines and software found in life. That is fair game. It is also fair game to make testable predictions about the future evolution of genomes. If genomes on average are deteriorating, then this casts doubt on evolutionary claims. That is science. Whether God made life? That seems a little outside of science personally, although Richard Dawkins thinks such questions are in the domain of science. So if I cited Dawkins, one could say ID is a legitimate scientific enterprise, but I prefer to be more circumspect as I argue (like Stephen Meyer) that this is a question that is less important in the scheme of things, and will be moot if life is indeed designed.
Again, to me, ID is a forensic hypothesis that draws upon scientific knowledge. Whether it is science is not a question I try to answer in the affirmative. Electromagnetism is science.
That said, how would I answer regarding my hostility toward mindless OOL and various evolutionary theories? I expressed skepticism that 2nd law can be used against OOL. On the other side, mindless OOL proponents have yet to even demonstrate in principle that physical law reasonable boundary conditions makes the emergence of the computers found in life to be even reasonably probable. One does not necessarily have to invoke God to at least concede that perhaps mechanisms no longer accessible and very dissimilar to anything we know originated life. But even such modest claims or modest skepticism of mindless OOL are greeted as anti-science.
Finally, it is certainly reasonable to express interest in the mechanical details of how things like a 3-chambered heart can rewire itself into a 4-chambered heart without killing the species. Looking at fossils skeletons (that have no informative soft tissue) and DNA sequences aren’t sufficient to make the mechanical questions disappear. And they are not minor questions.
Take the sonar of the whale or bat. I meet ID proponents who worked on sonar systems in the navy. When they hear evoltuionary biologists make explanations of how sonar evolved based on phylogenies, the explanations in favor of evolution sound about as bad (if not far worse) than ID proponents hand waving the 2nd law to explain design. The mechanical details are missing. The conclusions do not follow from the premises.
Even if the ID proponents are ultimately wrong, I can’t fault them for criticizing the lack of due process according to the standards that other disciplines are held to.
Now as far as the Wedge, and politics, let’s assume for the sake of argument its all about nefarious intentions. If evolutionary theory were as well established as electromagnetism, all the politics in the world would be hard pressed to overturn the theory. The reason this problem persists is that evolutionary theory (even if true) simply cannot support its claims on evidential grounds, not now, or maybe ever.
And to the extent that evolutionary biologists make claims that seem to fly in the face of engineering and operational biology, they can expect to be challenged. Even if they are ultimately right, their case has not been demonstrated to the same standards as other scientific theories.
But one final word about politics. How does that explain the breaking of ranks by individuals like Richard Sternberg, Dean Kenyon, John Sanford, Robert Marks or even Michael Behe? They have little to gain and much to lose for joining the ID side. Even if they are wrong, it seems to me they are sincere.
I know about the Wedge. I was at a luncheon and I met Howard Ahmanson in 2005. Rick Santorum came by and offered a few words. I get no money or increased career opportunities for being in this debate, nor do I intend to. I’m in the discussion because I find the topic interesting and I enjoy debates.
I was an alumni of the schools where Caroline Crocker taught. She has no job since she joined the ID movement. Where is the profit for her and her family? It seems to me, her motives were sincere. Even supposing someone is wrong (and I don’t think Dr. Crocker is wrong, but for the sake of argument, let us assume that), it does not make them dishonest, only mistaken. And from all that I see, there are certainly people who are sincere and who are scientists that are in the ID movement. So I don’t believe it is all about politics, and even it were, it is irrelevant to whether various evolutionary theories are true.
The hypothesis of magical causation of historical events is anti-scientific in the same sense that it would be anti-scientific for a detective to propose that a murder was committed by ghosts.
Naturalistic hypotheses demand and lead to research into regularities. Magical thinking has no need for causes and discourages research of the kind conducted by Szostak.
Your sympathy for ID/creationism doesn’t change its well-documented history; nor does it change the fact that ID/creationist notions about fundamental concepts in science are egregiously wrong. They are so wrong that the language of ID/creationism grates like nails on a blackboard for those who really do understand the concepts and can see the research paths ahead.
What grates even more is the fact that ID/creationists, with their sensitive egos and lust for celebrity, want to throw their misconceptions and misrepresentations into the learning paths of young students who have the potential to push those frontiers of science in the future. The public school systems in the US are generally a poorly financed and poorly supported mess, with teachers becoming political scapegoats in nearly every major political election.
ID/creationism, as you well know, contributes to this mess; and those illegitimate organizations like the DI, AiG, and the ICR are a big part of that mess in many states. Those organizations have not earned a placed in the science curriculum, yet they try to bypass every quality control and peer review check to get directly to other people’s children. I consider that dishonest, verging on criminal.
You yourself barely understand enough science and thermodynamics to sustain any criticism of Sewell; you will not be able to hold out against your “cohorts” over at UD. You simply don’t know just how bad and how wrong Sewell and the other ID/creationists who push this crap really are; and you still retain serious misconceptions and misrepresentations you have picked up from that community. .
It doesn’t stop at physics and chemistry. Ever since Morris and Gish, ID/creationist leaders have spent all their time ensconced in their ideological organizations deliberately misinforming their followers daily about everything that is going on in science. You know very well that we can link to any ID/creationist website and see that going on at this very moment.
It is simply not possible to explain science to people who live in the echo chamber of misconceptions and misrepresentations that drown out everything else in your ID/creationist subculture.
Scientists no longer find arguing with ID/creationist useful. There are thousands of young students out there who really want a proper education in science; they don’t deserve to be sidetracked by a subculture of losers and science haters who will never make the effort to learn anything themselves.